Abstract
Objective
To evaluate the utility of high-resolution vessel wall imaging (HR-VWI) of middle cerebral artery (MCA), and to compare HR-VWI findings between striatocapsular infarction (SC-I) and lenticulostriate infarction (LS-I).
Materials and Methods
This retrospective study was approved by the Institutional Review Board, and informed consent was waived. From July 2009 to February 2012, 145 consecutive patients with deep subcortical infarctions (SC-I, n = 81; LS-I, n = 64) who underwent HR-VWI were included in this study. The degree of MCA stenosis and the characteristics of MCA plaque (presence, eccentricity, location, extent, T2-high signal intensity [T2-HSI], and plaque enhancement) were analyzed, and compared between SC-I and LS-I, using Fisher's exact test.
Results
Stenosis was more severe in SC-I than in LS-I (p = 0.040). MCA plaque was more frequent in SC-I than in LS-I (p = 0.028), having larger plaque extent (p = 0.001), more T2-HSI (p = 0.001), and more plaque enhancement (p = 0.002). The eccentricity and location of the plaque showed no significant difference between the two groups.
Conclusion
Both SC-I and LS-I have similar HR-VWI findings of the MCA plaque, but SC-I had more frequent, larger plaques with greater T2-HSI and enhancement. This suggests that HR-VWI may have a promising role in assisting the differentiation of underlying pathophysiological mechanism between SC-I and LS-I.
Deep subcortical infarction involving the basal ganglia, corona radiata, and/or internal capsule is often categorized into larger subcortical infarction (striatocapsular infarction [SC-I]) and smaller lacunar infarction (lenticulostriate infarction [LS-I]) (1). Being a large artery disease (LAD), occlusion of perforating branches by an atherosclerotic plaque in the middle cerebral artery (MCA) is a prevalent cause of SC-I (23). Conversely, LS-I is also classified as a small-vessel occlusive disease (SVO), related to small-vessel lipohyalinosis (34). The differentiation between SC-I and LS-I is clinically important, since differing pathogenesis of SC-I and LS-I necessitates different clinical managements; systemic anti-atherosclerotic medication, such as statin, may be effective in patients with SC-I, whereas blood pressure management with anti-platelet agents may be useful in patients with LS-I (5). However, LAD may also cause small LS-I due to the protrusion of an atherosclerotic plaque into the orifice of the perforators, occluding the lumen (4678910). Accordingly, deep subcortical infarctions with different vascular pathologies may have similar shape and location. Therefore, differentiating between SC-I and LS-I as the pathogenesis of LAD and SVO, is challenging (1).
High-resolution vessel wall imaging (HR-VWI) is a useful tool to image intracranial arteries, including MCA (1112131415). It is able to determine the mechanism of stroke, such as atherosclerosis, plaque rupture, and plaque growth over the perforator ostia (16, 17). In 2015, Ryoo et al. (16) applied HR-VWI to differentiate the vascular pathology in deep subcortical infarctions. Deep subcortical infarctions were classified as branch occlusive disease (BOD) (where parent arterial disease occludes the perforator's orifice), and the non-BOD (caused by artery-to-artery embolism). They discovered the characteristic plaque remodeling pattern, location and enhancement in the BOD groups compared to the non-BOD groups (16). However, based on the above classification, the BOD and non-BOD were comprised solely of LAD, and not SVO. Moreover, since BOD and non-BOD were defined by the location of the infarction on magnetic resonance imaging (MRI) (16), the BOD group could have included patients with underlying SVO. Therefore, this study had limitations in the differentiation between LAD and SVO in deep subcortical infarction confined to the striatocapsular region.
To overcome this limitation, our study divided the patients with deep subcortical infarctions into two groups: the SC-I group and the LS-I group. The concept of adopting concept of SC-I and LS-I was to include deep subcortical infarctions developing from both LAD and SVO, and to better assist in the differentiating the underlying stroke mechanism in LAD and SVD. With the use of HR-VWI, we aimed to evaluate the characteristic findings of MCA plaque in patients with deep subcortical infarctions, further distinguishing the difference between SC-I and LS-I. To summarize, our study aimed to evaluate the utility of HRVWI of MCA, and to compare the HR-VWI findings between SC-I and LS-I.
This retrospective study was approved by the Institutional Review Board, and informed consent was waived. From July 2009 to February 2012, 207 consecutive patients (124 men, 83 women; mean age, 62.3 years; range, 25–87 years) with acute and/or chronic infarctions in the MCA region, underwent HR-VWI for the evaluation of MCA. Among them, 145 patients (87 men, 58 women; mean age, 62.5 years; range, 30–86 years) with deep subcortical infarctions were included in this study. Patients with multiple lesions on diffusion-weighted imaging (DWI), including cortical infarctions attributed to embolism, with evidence of cardiac thrombus or arteriovenous shunt, ipsilateral carotid stenosis, total occlusion of MCA, or underlying vasculopathy other than atherosclerosis (dissection, vasculitis, Moyamoya disease, etc.), were excluded from this study. Clinical information was obtained by reviewing the electronic medical records. The demographic data, including age, sex, and medical history related to the risk factors of stroke (i.e., hypertension, diabetes, hyperlipidemia, cardiac arrhythmia, coronary artery disease, previous stroke history, smoking history) were acquired.
MRI was performed using a 3T scanner (Achieva; Philips Healthcare, Best, The Netherlands) with 8-channel (n = 1) and 32-channel (n = 144) SENSE head coils. During the study period, HR-VWI was implemented as a part of routine brain MRI protocol for patients with acute or chronic MCA region infarction. HR-VWI was obtained in patients with MCA infarction to evaluate possible parent artery disease on MCA, including in patients with normal-looking MCA on MR angiography (MRA) (181920). Acute infarction was defined based on DWI and apparent diffusion coefficient (ADC) map (21). For patients with acute infarctions, HR-VWI was not performed in the initial MRI, but was performed during the follow-up brain MRI within 7 days. Chronic infarction was defined in patients who had followup MRIs, and who were diagnosed with acute infarction more than 1 month before study inclusion. Patients with chronic infarction underwent HR-VWI as a part of routine follow-up MRI. A 3D time-of-flight (TOF) MRA was obtained for the localizer before HR-VWI. The HR-VWI was composed of T1-weighted imaging (T1WI), proton density imaging, T2-weighted imaging (T2WI), and contrast-enhanced T1WI (CE-T1WI). The acquisition orientations were sagittal for HR-VWI. The parameters for each sequence in HR-VWI are described in Table 1. In addition, DWI, conventional T1WI and T2WI, fluid-attenuated inversion recovery imaging, gradient recoiled echo T2*WI, and CE-T1WI were performed, as per the routine protocol of our institution for patients with clinically suspected stroke. The total scan time for the entire scanning process, including routine brain MRI and HR-VWI, was approximately 50 minutes.
Two board-certified neuroradiologists (with 7 and 17 years of experience, respectively), were blinded to the clinical information, and reviewed the HR-VWI, brain MRI and MRA, to reach a final consensual diagnosis.
Striatocapsular infarction and LS-I were classified by radiological characterization (114). SC-I was defined as a comma-shaped infarction with a diameter greater than 15 mm, restricted to the territory of the lenticulostriate arteries (LSAs). The location of SC-I included the caudate head, putamen, intervening internal capsule, and corona radiata. LS-I was defined as a solitary infarction attributable to single perforating artery occlusion. The upper diameter limit of LS-I was 15 mm, and the volume on DWI was less than 1.8 × 103 mm3. Both SC-I and LS-I were additionally classified into acute and chronic infarctions, in accordance with the signal intensity on DWI and ADC map (21).
Based on TOF-MRA, the degree of MCA diameter stenosis was classified as follows: none, 0%; mild, 0–49%; and moderate to severe, 50–99%. The reference vessels with normal diameter located contralateral or proximal to the stenosis determined the degree of stenosis (16). HR-VWI determined the presence of the plaques at ipsilateral MCA with infarction. A plaque was defined as an eccentric or focal wall thickening compared with the nearby vessel wall (1322). In cases with present MCA plaques, the eccentricity was defined as a localized plaque surrounding less than 75% of the vessel wall (23). The location of the plaque was then determined, using the thickest part of the plaque as a reference point on cross-sectional imaging. The MCA was segmented into 6 divisions in the short-axis view (antero-superior, superior, postero-superior, antero-inferior, inferior, and postero-inferior divisions), and the plaque location was designated as the divisions occupied by the plaque. Accordingly, one plaque could be distributed across the divisions. After localization, the plaque extent was also measured in the short-axis view. Among the 6 divisions designated for the plaque location, the number of divisions occupied by the plaque in each case was used to evaluate the plaque extent. Moreover, visual assessment corroborated whether the plaque was located on the origin of LSA. To locate the LSA origin, HR-VWI first located the infarction and its proximal apex. From the apex, down to the M1 segment of the ipsilateral MCA, we identified the vascular structure of LSA and its origin from the M1 segment. Finally, we evaluated the presence of intraplaque T2-high signal intensity (T2-HSI) and plaque enhancement. Intraplaque T2-HSI was interpreted with a reference to the signal intensity of gray matter in the adjacent brain parenchyma (23). Plaque enhancement was identified when signal intensity increased by more than 20% after contrast agent injection (16).
Categorical variables are expressed as frequencies with percentages, and continuous variables are presented as the mean values ± standard deviations. Clinical data between patients with SC-I and LS-I were compared using Student's t test, chi-square test, and Fisher's exact test. According to the presence of acute or chronic infarctions, the characteristics of MCA plaque between the two groups were compared using chi-square test and Fisher's exact test. The number of divisions reflecting the plaque extent was compared using Student's t test. In addition, we designated the findings of eccentric plaque, plaque on the LSA origin, intraplaque T2-HSI, and plaque enhancement, as the positive findings for symptomatic MCA plaque (1314232425). We divided 145 patients according to the presence and the absence of symptomatic plaque characteristics, and compared the incidence of SC-I and LS-I between the two groups. p values of less than 0.05 were considered statistically significant. Statistical analyses were performed using the SPSS software (version 19.0; SPSS Inc., Chicago, IL, USA).
A total of 81 patients with SC-I and 64 patients with LS-I were included in our study. Table 2 shows the demographic data and risk factors of stroke in patients with SC-I and LSI. No statistically significant difference was observed with respect to the clinical data between the two groups.
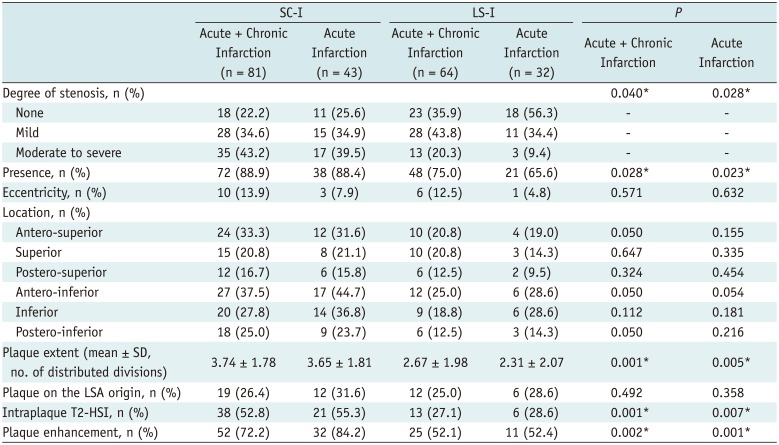
Table 3 summarizes the radiologic findings of MCA plaques of SC-I and LS-I. Totally, TOF-MRA revealed that 63 (77.8%) of 81 patients with SC-I, and 41 (64.1%) of 64 patients with LS-I, had stenosis in MCA. The stenosis was more severe in SC-I than in LS-I (p = 0.040). On HR-VWI, the plaque in MCA could be identified in 72 out of 81 patients with SC-I (88.9%), and in 48 out of 64 patients with LS-I (75.0%, p = 0.028). Among those with MCA plaque, the frequency of eccentric plaque showed no significant difference between the two groups (13.9% vs. 12.5%, respectively; p = 0.571). The location of the plaque according to the 6 divisions in the short-axis view was also not significantly different between SC-I and LS-I (all divisions, p > 0.05) (Fig. 1). The number of divisions in which the plaques were distributed was significantly higher in SC-I (3.74 ± 1.78) than in LS-I (2.67 ± 1.98, p = 0.001). The presence of the plaque on the origin of LSA did not significantly differ between the two groups (p = 0.492). The MCA plaque in SC-I showed more T2-HSI (52.8%) and more plaque enhancement (72.2%) than in LS-I (27.1% and 52.1%, p = 0.001 and 0.002, respectively). The same trend in the statistical result was derived in the analysis including only those patients with acute infarction (Table 3).
When we compared the incidence of SC-I and LS-I according to the presence and the absence of symptomatic MCA plaque characteristics, 141 patients with at least one symptomatic plaque characteristic showed a higher incidence of SC-I (n = 73) than LS-I (n = 48, p = 0.023). Figure 2 is a representative case of SC-I with symptomatic MCA plaque findings.
Our results revealed that patients with SC-I had more severe stenosis at MCA than patients with LS-I. According to HR-VWI, the MCA plaque was more frequent and extensive in SC-I than LS-I. More importantly, intraplaque T2-HSI and plaque enhancement were more frequently detected in SC-I than in LS-I. Therefore, HR-VWI provided characteristic MCA plaque findings, and could help to differentiate between SC-I and LS-I.
Many studies have utilized HR-VWI to determine the mechanism of ischemic stroke by revealing the underlying MCA plaque morphology in deep subcortical infarctions (111213141626). Deep subcortical infarctions could develop as LAD from atherosclerosis in MCA, or as SVO from perforator occlusion (12347927). One previous study classified deep subcortical infarctions from LAD into either BOD by parent artery disease causing perforator orifice occlusion, or non-BOD by artery-to-artery embolism, in accordance with the presence or absence of infarction outside the striatocapsular region, such as cortical infarction (16). As a result, BOD was associated with milder stenosis, more frequent positive remodeling, as well as more frequent and more eccentric plaque enhancement in MCA than non-BOD. However, patients with deep subcortical infarctions from underlying SVO were not included in this study. Nevertheless, since deep infarctions confined at the striatocapsular region can also result from SVO, the definition of BOD can be limited to reflect the underlying pathology of LAD.
To overcome this limitation, we adopted the classification of SC-I and LS-I in deep subcortical infarctions. We believe that the differentiation between LAD and SVO in deep subcortical infarctions without evidence of distal embolism, is also important in patient management. SC-I refers to the infarctions restricted to the basal ganglia and corona radiata which do not extend to the overlying cortex, with typical shape and clinical features (4927). SC-I may result from atherosclerotic stenosis of the ipsilateral carotid artery or MCA, or from cardiac or artery-to-artery embolism, rather than small vessel lipohyalinosis (1379). Accordingly, the size of SC-I is considered to be greater than 2–3 cm (127), with severe clinical symptoms including hemiparesis, aphasia, neglect, or apraxia (). Conversely, LS-I is a typical small deep lacunar infarction resulting from arteriolosclerosis of the small penetrating arteries by lipohyalinosis and angionecrosis (19). Therefore, LS-I can be determined by its small size–usually smaller than 1.5 cm (13)–and its benign clinical course (1). However, small deep subcortical infarctions can sometimes result from LAD or cardioembolism rather than SVO, showing similar size and symptom with LS-I, while at the same time showing a pathogenesis similar to SC-I (1). As a result, SC-I and LS-I can share similar morphological features and clinical manifestations, making it difficult to differentiate the underlying vascular pathology in intermediate-sized deep subcortical infarction (1).
In our study, we employed HR-VWI to compare the characteristic findings of MCA plaques between patients with SC-I and LS-I. Overall, HR-VWI revealed MCA plaque in 72 of 81 patients with SC-I, and in 48 of 64 patients with LS-I. There were more patients who showed atherosclerotic MCA plaque on HR-VWI than those who demonstrated luminal stenosis on TOF-MRA (63 SC-I, 41 LS-I). This mismatch can be explained to some degree, as follows. First, the resolution of TOF-MRA was lower than that of HR-VWI. The voxel size for TOF-MRA was 0.5 × 0.35 × 1.2 mm (0.21 mm3), while that for HR-VWI was 0.5 × 0.2 × 1 mm (0.1 mm3). This probably allowed HR-VWI to delineate finer structures in MCA than in TOF-MRA. Second, previous studies have already reported that HR-VWI could reveal abnormal wall thickening in the patent artery which appear to be normal on TOF-MRA (181920). It has been concluded that this phenomenon may be due to arterial adaptation to plaque development, causing positive arterial remodeling (1829).
Notably, MCA stenosis on TOF-MRA was more severe, and the plaque on HR-VWI was more frequent, with greater extent in SC-I patients than in LS-I patients. This demonstrates that atherosclerosis of MCA is a more frequent cause of SC-I than of LS-I. Importantly, the parameters for vulnerable plaque, including T2-HSI and plaque enhancement (14232425), were more frequently detected in SC-I than in LS-I. Therefore, the vulnerable MCA plaque also contribute to the development of infarction with an underlying pathology of LAD. In 2012, Kim et al. (14) determined the infarction pattern using HR-VWI, and they discovered that 1) the vulnerable plaque was frequently associated with artery-to-artery embolic infarction, 2) stable plaque was more associated with in situ thrombosis or SVO, and 3) patients without plaque mostly had SVO. These findings are in line with our results. Here, we show that the plaque is more extensive in patients with SC-I than in patients with LS-I. However, our results suggest that vulnerable MCA plaque is capable of creating SC-I, without the development of cortical artery-to-artery embolic infarctions. Hence, we can assume that the incidence of vulnerable plaque may also be high in SC-I, without any evidence of distal embolic infarction. Indeed, when we compared the number of SC-I and LS-I according to the presence of symptomatic MCA plaque on HR-VWI (1314232425), the incidence of SC-I was higher in patients who had symptomatic plaque findings.
Imaging findings of patients who only had acute infarctions at the time of HR-VWI were analyzed separately. The purpose was to overcome the limitation of the temporal discrepancy between the onset time of infarction and the imaging time. According to the analysis, cases with both acute and chronic infarctions show a similar trend to those with only acute infarctions. It is verified that the imaging time of HR-VWI did not significantly affect the result of our study.
Although the plaque extent and plaque vulnerability assessed by T2-HSI and plaque enhancement (14232425) were larger in SC-I than in LS-I, we should carefully consider the sizeable proportions of LS-I cases showing similar features to SC-I. Especially regarding plaque vulnerability, 20.3% of acute and chronic LS-I showed intraplaque T2-HSI, and 39.1% of them showed plaque enhancement. In 2012, Chung et al. (20) performed HR-VWI in patients with LS-I, and they reported a surprisingly high prevalence of parent artery branch atheromatous plaques. In accordance with this previous report, our study suggests that small-sized LS-I develop from symptomatic plaque in MCA, which should therefore be categorized as LAD, and not SVO. Likewise, we were unable to detect any MCA plaque in 11.1% of acute and chronic SC-I. In fact, the occlusion of perforating artery causes infarction with a maximal diameter of up to 3 cm (1). This indicates that categorizing SC-I as LAD, and LS-I as SVO, solely based on size criteria can be incorrect and misleading for the clinical management of the patient. Therefore, we recommend that HR-VWI should be used to precisely assist in the subtyping of SC-I and LS-I in deep subcortical infarction.
Regarding the plaque location, a previous study reported that superior wall plaques near the orifice of LSA were more likely to be symptomatic, resulting in larger penetrating artery infarction (1326). However, our results showed that the location of the plaque did not significantly differ between SC-I and LS-I. We believe that this was due to the limitation of the assignment of plaque location on the short-axis views. On longitudinal morphological analysis, the plaque can be divided into mid-point and shoulder area, where the plaque shoulder is more prone to rupture (303132). Based on this, we assume that if the inferiorly-located plaque is large enough to place its shoulder at the LSA origin, it can cause LSA occlusion and symptomatic infarction by plaque rupture. Indeed, our results showed that plaque extent was significantly larger in SC-I than in LS-I, thereby supporting the above explanation. Hence, we speculate that plaque location could not be used in differentiating between SC-I and LS-I. Rather, under the presence of the MCA plaque, the focus should be on the evaluation of plaque vulnerability on HR-VWI, for good patient management. In addition, LSA itself is still limited to be visualized on 3T MRI, since it has a small diameter ranging from 0.3 to 0.7 mm (3334). A future progress in the resolution of HR-VWI or the utility of 7T MRI (33) may enable a proper evaluation of the relationship between MCA plaque location and LSA origin.
Our study has some limitations. First, the atherosclerotic plaques in MCA were not histopathologically confirmed. However, since all patients in our study survived infarction, the pathological confirmation was impossible. Second, as mentioned before, the resolution was not sufficient to provide a clear evaluation of LSA itself. The visualization of LSA and its orifice at MCA would provide substantial information regarding vascular pathology, and concrete evidence for the development of SVO. Therefore, further efforts should be made to improve the resolution of HR-VWI. Third, we evaluated the plaque extent by dividing MCA into 6 divisions, and calculated the number of divisions occupied by the plaque. A measurement of the plaque volume would have been a more accurate approach, but we believe that this was a convenient method to estimate the axial extent of the MCA plaque. Lastly, our scan range was approximately 16 mm, which may not have covered the entire length of the M1 segment of MCA. However, since the main purpose of our study was to evaluate the plaque in the M1 segment that contributes to the development of the deep subcortical infarction, we pre-adjusted the scan range of the M1 segment for each individual patient to include the LSA origin, by confirming the proximal portion of the infarction on DWI and by rough tracing the signal of LSA on the source images for TOF-MRA. After approximating the LSA origin on the M1 segment, more than 50% of the M1 segment was encompassed, with certitude of the inclusion of the LSA origin.
In conclusion, both SC-I and LS-I have similar HR-VWI findings of the MCA plaque, but SC-I had more frequent, larger plaques with greater T2-HSI and plaque enhancement. Therefore, HR-VWI may have a promising role in assisting in the differentiation of underlying mechanism between SC-I and LS-I.
References
1. Jung S, Hwang SH, Kwon SB, Yu KH, Lee BC. The clinico-radiologic properties of deep small basal ganglia infarction: lacune or small striatocapsular infarction? J Neurol Sci. 2005; 238:47–52. PMID: 16126229.

2. Boiten J, Lodder J. Large striatocapsular infarcts: clinical presentation and pathogenesis in comparison with lacunar and cortical infarcts. Acta Neurol Scand. 1992; 86:298–303. PMID: 1414250.

4. Donnan GA, Bladin PF, Berkovic SF, Longley WA, Saling MM. The stroke syndrome of striatocapsular infarction. Brain. 1991; 114(Pt 1A):51–70. PMID: 1998890.
5. Nah HW, Kang DW, Kwon SU, Kim JS. Diversity of single small subcortical infarctions according to infarct location and parent artery disease: analysis of indicators for small vessel disease and atherosclerosis. Stroke. 2010; 41:2822–2827. PMID: 20966406.
6. Bogousslavsky J. The plurality of subcortical infarction. Stroke. 1992; 23:629–631. PMID: 1579957.

7. Levine RL, Lagreze HL, Dobkin JA, Turski PA. Large subcortical hemispheric infarctions. Presentation and prognosis. Arch Neurol. 1988; 45:1074–1107. PMID: 3052374.
8. Caplan LR. Intracranial large artery occlusive disease. Curr Neurol Neurosci Rep. 2008; 8:177–181. PMID: 18541112.

9. Fisher CM. Capsular infarcts: the underlying vascular lesions. Arch Neurol. 1979; 36:65–73. PMID: 420625.
10. Lhermitte F, Gautier JC, Derouesné C. Nature of occlusions of the middle cerebral artery. Neurology. 1970; 20:82–88. PMID: 5460773.

11. Zhao DL, Deng G, Xie B, Ju S, Yang M, Chen XH, et al. High-resolution MRI of the vessel wall in patients with symptomatic atherosclerotic stenosis of the middle cerebral artery. J Clin Neurosci. 2015; 22:700–704. PMID: 25744074.

12. Xu WH, Li ML, Gao S, Ni J, Yao M, Zhou LX, et al. Middle cerebral artery intraplaque hemorrhage: prevalence and clinical relevance. Ann Neurol. 2012; 71:195–198. PMID: 22367991.

13. Xu WH, Li ML, Gao S, Ni J, Zhou LX, Yao M, et al. Plaque distribution of stenotic middle cerebral artery and its clinical relevance. Stroke. 2011; 42:2957–2959. PMID: 21799160.

14. Kim JM, Jung KH, Sohn CH, Moon J, Han MH, Roh JK. Middle cerebral artery plaque and prediction of the infarction pattern. Arch Neurol. 2012; 69:1470–1475. PMID: 22910889.

15. Niizuma K, Shimizu H, Takada S, Tominaga T. Middle cerebral artery plaque imaging using 3-Tesla high-resolution MRI. J Clin Neurosci. 2008; 15:1137–1141. PMID: 18703337.

16. Ryoo S, Lee MJ, Cha J, Jeon P, Bang OY. Differential vascular pathophysiologic types of intracranial atherosclerotic stroke: a high-resolution wall magnetic resonance imaging study. Stroke. 2015; 46:2815–2821. PMID: 26330443.

17. Bodle JD, Feldmann E, Swartz RH, Rumboldt Z, Brown T, Turan TN. High-resolution magnetic resonance imaging: an emerging tool for evaluating intracranial arterial disease. Stroke. 2013; 44:287–292. PMID: 23204050.
18. Li ML, Xu WH, Song L, Feng F, You H, Ni J, et al. Atherosclerosis of middle cerebral artery: evaluation with high-resolution MR imaging at 3T. Atherosclerosis. 2009; 204:447–452. PMID: 19041971.

19. Klein IF, Lavallée PC, Schouman-Claeys E, Amarenco P. High-resolution MRI identifies basilar artery plaques in paramedian pontine infarct. Neurology. 2005; 64:551–552. PMID: 15699395.

20. Chung JW, Kim BJ, Sohn CH, Yoon BW, Lee SH. Branch atheromatous plaque: a major cause of lacunar infarction (high-resolution MRI study). Cerebrovasc Dis Extra. 2012; 2:36–44. PMID: 23060895.

21. van Everdingen KJ, van der Grond J, Kappelle LJ, Ramos LM, Mali WP. Diffusion-weighted magnetic resonance imaging in acute stroke. Stroke. 1998; 29:1783–1790. PMID: 9731595.

22. Zou XD, Chung YC, Zhang L, Han Y, Yang Q, Jia J. Middle cerebral artery atherosclerotic plaques in recent small subcortical infarction: a three-dimensional high-resolution MR study. Biomed Res Int. 2015; 2015:540217. PMID: 26539508.

23. Chung GH, Kwak HS, Hwang SB, Jin GY. High resolution MR imaging in patients with symptomatic middle cerebral artery stenosis. Eur J Radiol. 2012; 81:4069–4074. PMID: 22846476.

24. Skarpathiotakis M, Mandell DM, Swartz RH, Tomlinson G, Mikulis DJ. Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke. AJNR Am J Neuroradiol. 2013; 34:299–304. PMID: 22859280.

25. Vergouwen MD, Silver FL, Mandell DM, Mikulis DJ, Swartz RH. Eccentric narrowing and enhancement of symptomatic middle cerebral artery stenoses in patients with recent ischemic stroke. Arch Neurol. 2011; 68:338–342. PMID: 21403018.

26. Yoon Y, Lee DH, Kang DW, Kwon SU, Kim JS. Single subcortical infarction and atherosclerotic plaques in the middle cerebral artery: high-resolution magnetic resonance imaging findings. Stroke. 2013; 44:2462–2467. PMID: 23847248.
27. Bladin PF, Berkovic SF. Striatocapsular infarction: large infarcts in the lenticulostriate arterial territory. Neurology. 1984; 34:1423–1430. PMID: 6493490.

28. Croisille P, Turjman F, Croisile B, Tournut P, Laharotte JC, Aimard G, et al. Striatocapsular infarction: MRI and MR angiography. Neuroradiology. 1994; 36:430–431. PMID: 7991084.

29. Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987; 316:1371–1375. PMID: 3574413.

30. Thrysøe SA, Oikawa M, Yuan C, Eldrup N, Klaerke A, Paaske WP, et al. Longitudinal distribution of mechanical stresses in carotid plaques of symptomatic patients. Stroke. 2010; 41:1041–1043. PMID: 20224059.

31. Toutouzas K, Karanasos A, Tsiamis E, Riga M, Drakopoulou M, Synetos A, et al. New insights by optical coherence tomography into the differences and similarities of culprit ruptured plaque morphology in non-ST-elevation myocardial infarction and ST-elevation myocardial infarction. Am Heart J. 2011; 161:1192–1199. PMID: 21641368.

32. Shindo S, Fujii K, Shirakawa M, Uchida K, Enomoto Y, Iwama T, et al. Morphologic features of carotid plaque rupture assessed by optical coherence tomography. AJNR Am J Neuroradiol. 2015; 36:2140–2146. PMID: 26272975.

33. Kang CK, Park CW, Han JY, Kim SH, Park CA, Kim KN, et al. Imaging and analysis of lenticulostriate arteries using 7.0-Tesla magnetic resonance angiography. Magn Reson Med. 2009; 61:136–144. PMID: 19097221.

34. Liem MK, van der Grond J, Versluis MJ, Haan J, Webb AG, Ferrari MD, et al. Lenticulostriate arterial lumina are normal in cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy: a high-field in vivo MRI study. Stroke. 2010; 41:2812–2816. PMID: 20966419.
Fig. 1
Location of MCA plaque in SC-I and LS-I.
Two circular graphs show relative incidence of MCA plaque in 145 patients. MCA is segmented into 6 divisions in short-axis view. Scale 1.0 (bright yellow) refers to highest incidence of plaque (100%), and scale 0.0 (dark red) refers to lowest incidence of plaque (0%). Incidence between 0% and 100% appears in gradient color display, from maximum and minimum values. Overall incidence of MCA plaque is higher in SC-I than in LS-I, but location of plaque among 6 divisions in MCA does not differ in SC-I and LS-I. AS = antero-superior, AI = antero-inferior, I = inferior, LS-I = lenticulostriate infarction, MCA = middle cerebral artery, PI = posteroinferior, PS = postero-superior, S = superior, SC-I = striatocapsular infarction
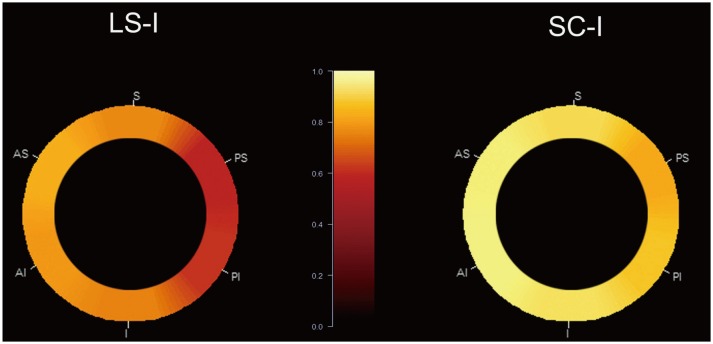
Fig. 2
70-year-old female with left SC-I.
A. DWI shows about 2.5 cm-sized acute SC-I in left basal ganglia and corona radiata. B. On TOF-MRA, left M1 segment of MCA shows luminal irregularity with mild stenosis (arrow). C-F. HR-VWI, including T1WI (C), PDI (D), T2WI (E), and CE-T1WI (F), demonstrates eccentric wall thickening of stenotic portion of left M1 segment, indicating atherosclerotic plaque (arrows). Note focal intraplaque T2-HSI on T2WI and contrast enhancement of plaque on CE-T1WI (arrows), suggesting plaque vulnerability. CE-T1W1 = contrast-enhanced T1WI, DWI = diffusion-weighted imaging, HR-VWI = high-resolution vessel wall imaging, MRA = MR angiography, PDI = proton density imaging, TOF = time-of-flight, T1W1 = T1weighted imaging, T2WI = T2-weighted imaging, T2-HSI = T2-high signal intensity
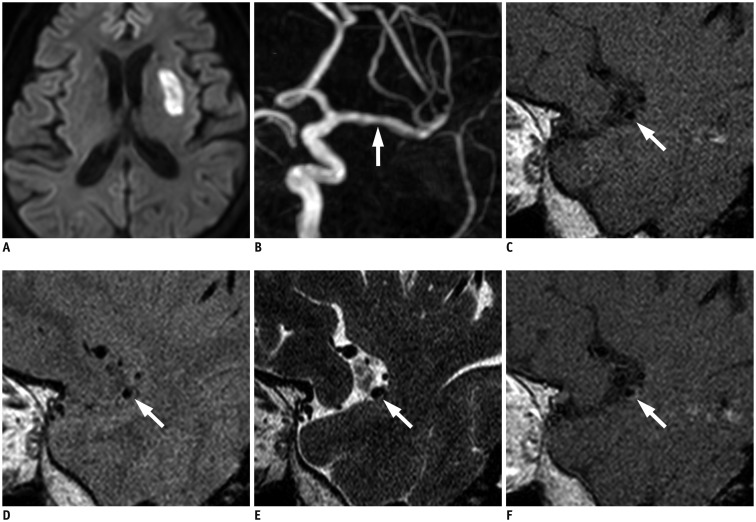
Table 1
Parameters for HR-VWI
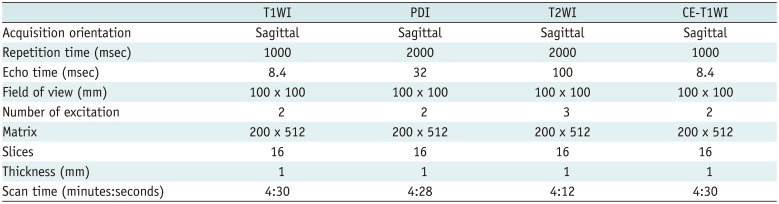
Table 2
Clinical Characteristics of 145 Patients According to Infarction Patterns
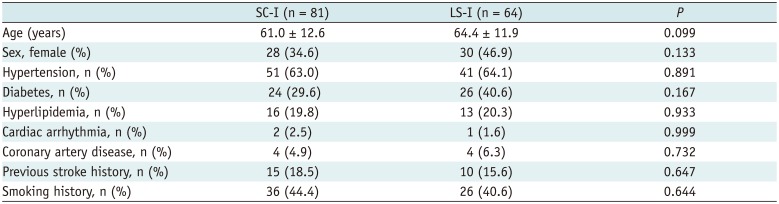
Table 3
Characteristics of MCA Plaque According to Infarction Patterns




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download