Abstract
Although a rare disease, anal cancer is increasingly being diagnosed in patients with risk factors, mainly anal infection with the human papilloma virus. Magnetic resonance imaging (MRI) with external phased-array coils is recommended as the imaging modality of choice to grade anal cancers and to evaluate the response assessment after chemoradiotherapy, with a high contrast and good anatomic resolution of the anal canal. MRI provides a performant evaluation of size, extent and signal characteristics of the anal tumor before and after treatment, as well as lymph node involvement and extension to the adjacent organs. MRI is also particularly helpful in the assessment of complications after treatment, and in the diagnosis for relapse of the diseases.
Primary neoplastic conditions of the anal canal are uncommon. Although their diagnosis and follow-up are traditionally based on clinical assessment, imaging, particularly MRI, plays an important role in staging and follow-up after treatment. In this article, we discuss and illustrate the current role of MRI in the pre and post-therapeutic management of these patients.
The anal canal extends from the anorectal junction to the anal margin, and is usually 3–6 cm in length. The anal margin is the circular rim of pigmented skin with folds surrounding the anus. The anal canal is composed of an internal sphincter and an external sphincter complex. The internal sphincter is the distal continuation of the inner circular rectal muscular layer, while the external sphincter complex includes the inferior confluence of the levator ani muscle, the puborectalis sling, and the deep, subcutaneous, superficial external sphincter muscles. Both sphincters are separated by the fatty intersphincteric space (Figs. 1, 2).
A significant marker of the anal canal is the dentate line, separating the upper part lined with transitional zone or rectal glandular mucosa, and the lower part (distal third) lined by nonkeratinizing squamous epithelium. Tumors located above the dentate line drain into the perirectal and internal iliac nodes, whereas tumors below the dentate line drain into the inguinal and femoral lymph nodes. The dentate line cannot be seen on MRI, but its position can be estimated by dividing the anal canal into thirds, so that the dentate line lies at the junction of the upper one-third and lower two-thirds. The current World Health Organization distinguishes invasive tumors as epithelial tumors (squamous cell carcinoma, adenocarcinoma, mucinous adenocarcinoma, small cell carcinoma, undifferentiated carcinoma), nonepithelial tumors (leiomyoma, gastrointestinal tumor, myofibroma), carcinoid tumors and melanomas. Virtually all anal canal cancers (AC) are squamous, either keratinizing or non-keratinizing, according to their origin below or above the dentate line, although with similar biological behaviour. As far as tumors near the anorectal junction are concerned, the American Joint Committee on Cancer (AJCC) recommends that tumors with an epicenter more than 2 cm superior to the dentate line should be staged as low rectal carcinomas, whereas the others tumors should be staged as AC (1). In practice, however, the precise point of origin is often uncertain at diagnosis.
Anal canal cancers are uncommon, with an incidence of 1.5:100000 persons per year (2). Their rate has been steadily increasing, due to the rising prevalence of the strongest risk factors, the human immunodeficiency virus (HIV), and human papilloma virus (HPV), mainly HPV-16 subtype (3,4,5). Although the overall incidence of AC remains higher among women, it is much higher in men who practice anoreceptive intercourse (4). AC is also strongly associated with immune suppression in transplant recipients (3). Other risk factors include cervical dysplasia, autoimmune disorders and cigarette smoking (4). Some reports have also shown an increased risk of anal cancer with long-standing, severe perianal fistulizing Crohn's disease (5,6,7). In these cases, AC occurs at a younger age. Diagnosis is often delayed, and at an advanced stage the prognosis is grave.
Diagnosis of these tumors is often delayed as they commonly present with bleeding that is generally attributed to hemorroids. They may also manifest as any combination of a mass, non-healing ulcer, pain, itching, discharge, fecal incontinence and fistula. The diagnosis of anal cancer is made on biopsy-proven histology. More advanced lesions in the distal anal canal may extend to the skin at the anal margin. Rarely, patients present with inguinal lymphadenopathy.
Anal margin tumors can be treated by surgical excision alone, as opposed to anal canal tumors, which have a worse prognosis and are treated with definitive chemoradiotherapy.
A multidisciplinary approach is mandatory. Large prospective validated randomized controlled trials (8,9,10,11,12) support the use of radiotherapy with a dose ranging between 45 and 50 Gy, combined with mitomycin-C and infusional 5-FU chemotherapy as the standard of care for all AC. This leads to complete tumor regression in 80–90% of patients, with locoregional failures of up to 15% (13). Standard radiation fields include the pelvis, anus, perineum, and inguinal nodes. Inguinal nodes receive a booster dose if there is evidence of gross nodal disease (14). The incidence of nodal involvement increases with increasing primary tumor size, and is at least 20% in patients with T3 disease (15). In case of surgical treatment, abdomino-perineal resection is performed for salvage in patients with locally recurrent disease, or for non-responsive patients with persistent tumors (13). Local excision may be performed in case of smaller lesions (< 2 cm in diameter), those involving the anal margin, and well differentiated lesions (13). In these cases, it is important to adequately stage the lesion in order to rule out the presence of positive nodes. Lastly, metastatic disease is considered incurable.
Most studies dealing with MRI of AC have been performed at 1.5T (14,15,16). No specific patient preparation is required. Although some teams use antispasmolytic agents to obtain good quality MR images (16,17,18,19), we did not find this a necessity. As for rectal cancer (20), insertion of a probe in the anal canal does not seem to be necessary either. Adequate coverage of the anal margin, inguinal areas and higher nodal stations, i.e., at least the sacral promontory and the area below the aortic bifurcation, should be obtained. Some authors also add T1-weighted MR sequences up to the renal hilum (21). MR protocols include high-resolution T2-weighted sequences along the three planes, with coronal and axial scans planned parallel and perpendicular to the long axis of the anal canal. Slice thickness for imaging the tumor is generally kept under 4 mm (16,17,18,19, 22, 23), with a small field of view.
Most authors (16,17,18,19,20) use additional short-tau inversion recovery sequences. Despite their limited spatial detail, these sequences are helpful for identifying fistula tracts present at the initial staging, or those developing during the treatment. We also use gadolinium-enhanced fatsuppressed T1-weighted sequences in at least one plane, in order to enhance delineation of the relationships of the tumor to the sphincters, and detect lesion enhancement, as has been previously reported (19, 14).
Diffusion-weighted imaging appears to have an emerging role (23), especially for differentiating suspected small residual/recurrent tumors from treatment-related changes; however, no dedicated studies on the potential added value of these MRI sequences have been published so far.
On diagnosis of primary anal cancer, the specific standard workup includes a digital rectal examination and anoscopy, inguinal lymph node assessment with biopsy of the nodes if indicated, and systemic radiologic evaluation. The latest European Society for Medical Oncology (ESMO) recommendations published in 2014 (13) advocates the use of phased-array MRI of the pelvis, or if not available, endoanal ultrasound (EUS). EUS has a limited scope compared with MRI, since it does not provide data on many of the regional nodal stations for anal cancer, and is operator dependent and unable to assess stenotic tumors. Owing to its small field of view, it is best reserved for small T1 lesions.
Computed tomography (CT) of the thorax and abdomen is used to assess distant extent of spread, mainly metastasis in liver and lungs, which is present in less than 10% of cases. 18-Fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT has an increasing role in staging and treatment planning of anal carcinoma. Up to 98% of anal tumors are FDG-avid. At diagnosis, FDG PET/CT is used to evaluate primary tumor size, lymph node status, and the presence of distant metastases. Several studies (24,25,26) report the increased sensitivity of FDG PET/CT compared with contrast-enhanced CT for detection of abnormal lymph nodes, especially for advanced T2 tumors, with a change in staging of the anal carcinoma in about 20% of the cases (27). However, these studies remain limited, because histological confirmation of FDG-avid nodes is lacking.
Sensitivity of MRI for the identification of AC has been reported to approach 90–100%, with high concordance regarding tumor size, compared to transanal endoscopic ultrasound (28). Position (anal canal, anal margin), circumferential tumor extent (position on clock), and subjective T2-weighted signal intensity (high-similar to fluid; low- similar to muscle and intermediate) need to be noted. Infiltration of adjacent organs (vagina, prostate), transsphincteric extension into the ischioanal/ischiorectal fossa, and the presence of perianal fistulas or abscesses are also recorded.
Anal cancers display high signal intensity relative to skeletal muscles on T2-weighted images, but lower than normal ischioanal fat, and low to intermediate signal intensity on T1-weighted images. They are markedly enhanced after intravenous gadolinum contrast injection (Fig. 3) (17,18,19, 29). Anal cancers can extend to the rectum with skip lesions seen at a distance from the primary site, involving low or even middle rectum. They may develop on condylomas, particularly in HIV and HPV positive patients (Figs. 4, 5).
Radiologists should be aware of the possibility of neoplastic changes in long-standing anorectal fistulas, and should be alerted by the presence of soft tissue within the fistula (30). In such cases, AC will generally display T2-weighted high signal intensity, due to colloid content and mesh like internal enhancement (Fig. 6).
Anal melanoma is rare, accounting for only 2% of all melanomas (31), and is generally homogeneous. Primary anal lymphomas are also very rare, and appear as a mass with focal and/or circumferential thickening of the anal canal, potentially extending into the rectum, mildly enhancing after contrast medium injection, and typically without luminal obstruction (32).
T stage corresponds to the size of the primary tumor (Table 1), assessed by measuring the tumor in its longest diameter on T2-weighted MR images (Figs. 7, 8, 9) (27), but does not take into account the extension to the sphincters. As far as stage T4 is concerned, the ESMO defined that the invasion of the following organs for anal canal carcinoma does not affect T4 staging: external and/or internal sphincter muscles, rectal wall, puborectalis muscle, levator ani muscle and vestibule for tumors originating from the anal margin. For anal margin carcinoma, a T4 lesion is defined by invasion of deeper structures such as the skeletal muscle or cartilage. Unlike N staging for rectal cancer, the location of nodal stations involved determines the N stage for anal cancer (15).
Proximal lymphatic drainage is to perirectal nodes along the inferior mesenteric artery. Infra-dentate and perianal skin tumors drain to the inguinal, femoral and external iliac nodes. Immediately above the dentate line, drainage is to internal pudendal nodes, and to the internal iliac system. All lymph nodes behind the external iliac vessels are considered to belong to the obturator fossa, and are thus a part of the internal iliac group. Nodes along the external and common iliac vessels are considered as distant spread. Criteria for diagnosis of lymph node metastasis are not validated, but enlargement (largest short- axis diameter > 1 cm for mesorectal nodes, and > 1.5 cm for other nodes), heterogeneity or necrosis, irregular contours and strong enhancement are considered to suggest involvement (Fig. 10) (21, 29).
Fifty per cent of recurrences occurring within the first 2 years post treatment are located around the primary site of disease, or as pelvic/inguinal lymph nodes (22). ESMO recommends clinical evaluation for complete response at 8 weeks post chemoradiotherapy (CRT), with a 3-to 6-monthly follow up thereafter for a 2-year period, stating that “MRI can capture and document response, but no individual MRI feature appears predictive of eventual outcome” (15). The use of imaging in patients following CRT is still debatable, and there is scarce data in the literature on assessment of tumor response using imaging modalities.
Other international recommendations do not endorse a role of MRI in post CRT surveillance (33, 34). However, these recommendations are based on series of very small sample size (17, 18, 29); in current practice, MRI often serves as the follow-up imaging modality (16, 17, 35). Although consensus criteria for response have not been described so far, reduction in tumor size, as well as in T2-weighted signal intensity of the treated tumor or associated lymphadenopathy, is indicative of response. The optimal timing for therapy assessment has so far not been defined, but size involution seems to be most evident at 6 months post treatment, and complete response has been reported to occur at around 26 weeks (18). Clinicians generally ask for the MRI to be performed 8 weeks after CRT completion, in accordance with what is established for rectal cancers. This time point is likely to be too early, as not all tumors will have achieved complete response, and also since inflammation is superimposed on the treated cancer.
A retrospective study by Kochhar et al. (16), comprising of 74 patients with non-metastatic anal squamous cell carcinoma with a demonstrable anal lesion on baseline staging treated with CRT undergoing 3 and 6 months MRI follow-up, has recently shown that tumor regression grade (TRG) 1/2 scores at 3 and 6 months had a 100% negative predictive value, and that TRG 4/5 scores at 6 months had a 100% positive predictive value. However, no imaging characteristic or TRG score independently prognosticated for late relapse or 3-year disease free survival (16). Reduction in size was seen at 3 (mean 28.79%) and 6 months (mean 81.39%), and reduction in signal intensity was recorded in 69% and 84% of patients at 3 and 6 months, respectively. Nodal disease was downstaged in 33/36 patients, with N0 status in 32 of these patients. The benefit of FDG-PET/CT studies seems to be detection of residual sub-clinical pelvic or extra-pelvic/para-aortic node involvement, rather than assessment of tumor response (13).
Radiation proctitis as well as fistula (Fig. 11) and abscesses may complicate radiotherapy. Radionecrosis is an uncommon event occurring in up to 10% of patients undergoing radiotherapy for anal cancer (36). It causes clinical (pain, anal stenosis, mucositis and diarrhea) and diagnostic problems. MRI finding of a hypointense area on T1/T2-weighted MR images with only a peripheral enhancement rim, is suggestive of this diagnosis (Fig. 12).
Recurrent disease is defined as initial complete response to therapy, with subsequent positive biopsies, more than six months after completion of treatment. Patients with recurrent disease may benefit from surgical salvage (13), and MRI is the best post- operative imaging modality. Similar to rectal cancers (37), T2-weightedhypointense signal at the site of the primary tumor after CRT, is a morphological sign consistent with response, as it represents fibrosis. However, MRI is unable to reliably exclude residual neoplastic foci within the fibrosis. Recently, Kochhar et al. (16) have described a novel ‘tram track’ sign on post-CRT scans, defined as parallel linear low signal at the inner and outer margin of the internal sphincter at the site of the original tumor. This sign had a negative predictive value for early local relapse of 83% at 6 months, and was present in more than half the patients (16). CT scan of thorax and abdomen (or PET/CT) is advised to rule out distant metastases. Tumors that are likely to relapse include tumors larger than 4 cm, with nodal involvement, and basaloid subtypes (23).
Figures and Tables
Fig. 1
Anatomy of anal canal.
T2-weighted coronal MR image. 1 = external sphincter, 2 = internal sphincter, 3 = ischioanal fossa, 4 = puborectal muscle, 5 = levator ani muscle
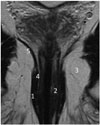
Fig. 2
Anatomy of anal canal.
Contrast-enhanced T1-weighted axial image. 1 = external sphincter, 2 = internal sphincter (high enhancement), 3 = fatty intersphincteric space
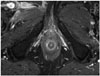
Fig. 3
MR signal of anal canal squamous cell carcinomas.
A, B. Axial (A) and coronal (B) T2-weighted MR images show high signal intensity tumor (arrow) relative to muscles, and of low signal intensity relative to normal ischioanal fat. Lesion is staged T2. C. On low b value of axial diffusion-weighted MR images, lesion shows hyperintensity (arrow). D. Lesion (arrow) is markedly enhanced on fat-suppressed contrast-enhanced T1-weighted axial MR image.
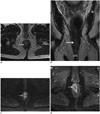
Fig. 4
Condyloma of anal canal in 53-year-old man.
Sagittal T2-weighted MR image showing condyloma (arrow) displaying cauliflower like appearance.
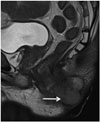
Fig. 5
Condyloma with high-grade dysplasia in 45-year-old man with HIV.
Anal margin lesion is seen on axial T2-weighted MR image (arrow, A). Same patient one year later (B, C), axial T2 (B) and fat-suppressed contrast-enhanced T1-weighted (C) MR images show increase in size of lesion (arrow, B) and heterogeneity of enhancement (arrow, C). Histological analysis concluded as squamous cell carcinoma developed on condyloma, T2N0M0.
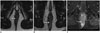
Fig. 6
Severe active perineal lesions and multiple fistulas in 50-year-old man with history of Crohn's disease.
Axial fat-suppressed gadolinium-enhanced T1-weighted MR image from first MRI in 2010 (A) shows complex fistula, transphicteric with extension to posterior part of right ischioanal fossa (arrow). Patient was lost to follow-up, and came back six years later. Axial T2 (B) and fat-suppressed gadolinium-enhanced (C) MR weighted images show fistula located at 6 o'clock (arrow, B) as well as large well-circumscribed high signal lesion on T2-weighted MR images (B), not to be misinterpreted as abscess, with inner peripheral enhancement (arrow, C), suggesting mucinous adenocarcinoma developed from long-standing fistula. Histological analysis of resected specimen confirmed this diagnosis.

Fig. 7
Squamous cell carcinoma of anal canal in 62-year-old man, T2N0M0.
Twenty-two mm lesion is seen invading internal and external sphincter, without extension to adjacent organs, on axial (arrow, A), T2-weighted, high b value (b 1000) (arrow, B), and fat-suppressed gadolnium-enhanced T1-weighted (arrow, C) MR images.

Fig. 8
Squamous cell carcinoma of anal canal, T4N1aM0, in 73-year-old woman.
Lesion has cranio-caudal extension of 85 mm, invading middle rectum, vagina and uterine cervix.
Axial T2 (A), sagittal T2 (B), and axial fat-suppressed contrast-enhanced T1-weighted MR image (C) show extension of lesion to vagina (white arrows, A, B), skip lesion to mid rectum (black arrow, B) and extension to uterine cervix (white arrow, C).
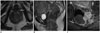
Fig. 9
Squamous cell carcinoma of anal canal, T4N0M1, in 60-year-old woman.
A. Coronal T2-weighted image shows squamous cell carcinoma of anal canal (arrow). B. Extension of lesion to left piriformis muscle (arrow).
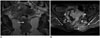
Fig. 11
Ano-vaginal fistula in 63-year-old woman, 6 months after chemoradiation therapy for T3N1cM0 squamous cell carcinoma of anal canal.
Axial T2 (A), and fat-suppressed contrast-enhanced T1-weighted MR (B) images display large fistula from treated anterior part of anal canal into vagina (arrows).

Fig. 12
63-year-old woman with squamous cell carcinoma of anal canal, T2N1aM0.
Twenty-five-mm lesion of anal canal shows high signal intensity on T2-weighted image (arrow, A), with strong enhancement on contrast-enhanced T1-weighted (arrow, B). MRI control after CRT shows lesion with peripheral enhancement on contrast-enhanced T1-weighted image (arrow, C), corresponding to radionecrosis.
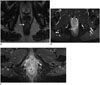
Table 1
TNM Classification, 8th Edition (27)

References
1. American Joint Committee on Cancer. AJCC cancer staging manual. 7th ed. New York: Springer;2010. p. 103–106.
2. Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010; 60:277–300.
3. Johnson LG, Madeleine MM, Newcomer LM, Schwartz SM, Daling JR. Anal cancer incidence and survival: the surveillance, epidemiology, and end results experience, 1973-2000. Cancer. 2004; 101:281–288.
4. Uronis HE, Bendell JC. Anal cancer: an overview. Oncologist. 2007; 12:524–534.
5. Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer. 2009; 124:2375–2383.
6. Barral M, Dohan A, Allez M, Boudiaf M, Camus M, Laurent V, et al. Gastrointestinal cancers in inflammatory bowel disease: an update with emphasis on imaging findings. Crit Rev Oncol Hematol. 2016; 97:30–46.
7. Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet. 1996; 348:1049–1054.
8. Peiffert D, Tournier-Rangeard L, Gérard JP, Lemanski C, François E, Giovannini M, et al. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: final analysis of the randomized UNICANCER ACCORD 03 trial. J Clin Oncol. 2012; 30:1941–1948.
9. Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB 3rd, Thomas CR Jr, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008; 299:1914–1921.
10. Flam M, John M, Pajak TF, Petrelli N, Myerson R, Doggett S, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol. 1996; 14:2527–2539.
11. Bartelink H, Roelofsen F, Eschwege F, Rougier P, Bosset JF, Gonzalez DG, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol. 1997; 15:2040–2049.
12. Sjödahl RI, Myrelid P, Söderholm JD. Anal and rectal cancer in Crohn's disease. Colorectal Dis. 2003; 5:490–495.
13. Glynne-Jones R, Nilsson PJ, Aschele C, Goh V, Peiffert D, Cervantes A, et al. Anal cancer: ESMO-ESSO-ESTRO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014; 25:Suppl 3. iii10–iii20.
14. Matalon SA, Mamon HJ, Fuchs CS, Doyle LA, Tirumani SH, Ramaiya NH, et al. Anorectal cancer: critical anatomic and staging distinctions that affect use of radiation therapy. Radiographics. 2015; 35:2090–2107.
15. Jones M, Hruby G, Stanwell P, Gallagher S, Wong K, Arm J, et al. Multiparametric MRI as an outcome predictor for anal canal cancer managed with chemoradiotherapy. BMC Cancer. 2015; 15:281.
16. Kochhar R, Renehan AG, Mullan D, Chakrabarty B, Saunders MP, Carrington BM. The assessment of local response using magnetic resonance imaging at 3- and 6-month post chemoradiotherapy in patients with anal cancer. Eur Radiol. 2017; 27:607–617.
17. Goh V, Gollub FK, Liaw J, Wellsted D, Przybytniak I, Padhani AR, et al. Magnetic resonance imaging assessment of squamous cell carcinoma of the anal canal before and after chemoradiation: can MRI predict for eventual clinical outcome? Int J Radiat Oncol Biol Phys. 2010; 78:715–721.
18. Koh DM, Dzik-Jurasz A, O’Neill B, Tait D, Husband JE, Brown G. Pelvic phased-array MR imaging of anal carcinoma before and after chemoradiation. Br J Radiol. 2008; 81:91–98.
19. Jederán É, Lõvey J, Szentirmai Z, Hitre E, Léránt G, Horváth K, et al. The role of MRI in the assessment of the local status of anal carcinomas and in their management. Pathol Oncol Res. 2015; 21:571–579.
20. Beets-Tan RG, Lambregts DM, Maas M, Bipat S, Barbaro B, Caseiro-Alves F, et al. Magnetic resonance imaging for the clinical management of rectal cancer patients: recommendations from the 2012 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting. Eur Radiol. 2013; 23:2522–2531.
21. Roach SC, Hulse PA, Moulding FJ, Wilson R, Carrington BM. Magnetic resonance imaging of anal cancer. Clin Radiol. 2005; 60:1111–1119.
22. Gourtsoyianni S, Goh V. MRI of anal cancer: assessing response to definitive chemoradiotherapy. Abdom Imaging. 2014; 39:2–17.
23. Kochhar R, Plumb AA, Carrington BM, Saunders M. Imaging of anal carcinoma. AJR Am J Roentgenol. 2012; 199:W335–W344.
24. Cotter SE, Grigsby PW, Siegel BA, Dehdashti F, Malyapa RS, Fleshman JW, et al. FDG-PET/CT in the evaluation of anal carcinoma. Int J Radiat Oncol Biol Phys. 2006; 65:720–725.
25. Mistrangelo M, Pelosi E, Bellò M, Ricardi U, Milanesi E, Cassoni P, et al. Role of positron emission tomography-computed tomography in the management of anal cancer. Int J Radiat Oncol Biol Phys. 2012; 84:66–72.
26. Winton Ed, Heriot AG, Ng M, Hicks RJ, Hogg A, Milner A, et al. The impact of 18-fluorodeoxyglucose positron emission tomography on the staging, management and outcome of anal cancer. Br J Cancer. 2009; 100:693–700.
27. Brierley JD, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 8th ed. Toronto: Wiley Blackwell;2016. p. 77.
28. Otto SD, Lee L, Buhr HJ, Frericks B, Höcht S, Kroesen AJ. Staging anal cancer: prospective comparison of transanal endoscopic ultrasound and magnetic resonance imaging. J Gastrointest Surg. 2009; 13:1292–1298.
29. Parikh J, Shaw A, Grant LA, Schizas AM, Datta V, Williams AB, et al. Anal carcinomas: the role of endoanal ultrasound and magnetic resonance imaging in staging, response evaluation and follow-up. Eur Radiol. 2011; 21:776–785.
30. Hama Y, Makita K, Yamana T, Dodanuki K. Mucinous adenocarcinoma arising from fistula in ano: MRI findings. AJR Am J Roentgenol. 2006; 187:517–521.
31. Singer M, Mutch MG. Anal melanoma. Clin Colon Rectal Surg. 2006; 19:78–87.
32. Rousset P, Hoeffel C. [Tumors of the rectum: MRI and CT features]. J Radiol. 2007; 88(11 Pt 1):1679–1687.
33. Benson AB 3rd, Arnoletti JP, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, et al. Anal carcinoma, version 2.2012: featured updates to the NCCN guidelines. J Natl Compr Canc Netw. 2012; 10:449–454.
34. Steele SR, Varma MG, Melton GB, Ross HM, Rafferty JF, Buie WD. Standards Practice Task Force of the American Society of Colon and Rectal Surgeons. Practice parameters for anal squamous neoplasms. Dis Colon Rectum. 2012; 55:735–749.
35. Renehan AG, O’Dwyer ST. Management of local disease relapse. Colorectal Dis. 2011; 13:Suppl 1. 44–52.
36. Borzomati D, Valeri S, Ripetti V, Vincenzi B, Rabitti C, Persichetti P, et al. Persisting perianal ulcer after radiotherapy for anal cancer: recurrence of disease or late radiation-related complication? Hepatogastroenterology. 2005; 52:780–784.
37. Hoeffel C, Mulé S, Laurent V, Pierredon-Foulogne MA, Soyer P. Current imaging of rectal cancer. Clin Res Hepatol Gastroenterol. 2015; 39:168–173.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download