Abstract
Objective
To investigate the diagnostic yield and accuracy of CT enterography (CTE) for early (< 12 postoperative months) surveillance of anastomotic recurrence after bowel resection for Crohn's disease (CD).
Materials and Methods
We analyzed 88 adults (60 males and 28 females; mean age, 31.4 ± 9.6 years) who underwent bowel surgery for CD that created ileocolic anastomosis without enteric stoma, and underwent CTE for surveillance of CD recurrence/aggravation within 12 post-operative months. The CD activity index (CDAI) at the time of CTE was < 150 (i.e., clinically silent) in 51 patients, and ≥ 150 in 37 patients. Diagnostic yields of CTE regarding CD recurrence in the ileocolic anastomosis and extraluminal penetrating complications were determined. CTE-related step-up therapy was recorded. These outcomes were compared between the two CDAI groups after accounting for major risk factors for CD recurrence. In a subgroup of 31 patients who underwent both CTE and ileocolonoscopy within 1 month, CTE accuracy for anastomotic recurrence was assessed using the Rutgeerts scoring as the reference standard.
Results
CTE diagnostic yield was 35.2% (31/88) for the anastomotic recurrence and 9.1% (8/88) for penetrating complications. 20.5% (18/88) of the patients underwent step-up therapy after CTE detection of anastomotic recurrence. These outcomes were not significantly different between CDAI < 150 and CDAI ≥ 150, except that CTE yield for extraluminal penetrating complications was significantly higher in CDAI ≥ 150 (16.2% [6/37] vs. 3.9% [2/51]; multivariable-adjusted p = 0.029). CTE showed 92.3% (12/13) sensitivity and 83.3% (15/18) specificity for anastomotic recurrence.
Recurrence of Crohn's disease (CD) after bowel surgery for CD is frequent, especially in the anastomotic region (i.e., the site of the anastomosis, and a short bowel segment proximal to it), even during the early postsurgical period (1234). Early recognition of recurrence is important because a delayed diagnosis can lead to irreversible bowel damage and another bowel surgery. The postsurgical recurrence of CD is often clinically silent: according to one systematic review (4), more than half of patients with recurrence within 1 year of surgery did not have remarkable symptoms. Accordingly, all CD patients are recommended to undergo ileocolonoscopic surveillance 6–12 months after bowel surgery for the early detection of recurrence (1256). In support of this recommendation, a recent randomized clinical trial (the POCER trial) showed that early routine ileocolonoscopy six months after bowel surgery for CD and appropriate treatment step-up in the case of endoscopic recurrence could improve patient outcomes (7).
Unlike endoscopy, the utility of cross-sectional enterography, including CT enterography (CTE) and MR enterography (MRE), for surveillance specifically within 12 months of surgery, remains to be clarified, although these tests are already available in clinical practice to examine postsurgical CD patients, with a recent survey (8) finding that CTE was preferred by surgeons. One critical reason for the uncertainty regarding the use of CTE or MRE for early postsurgical surveillance is a lack of studies, either prospective or retrospective, that directly and specifically investigate the tests in the early postsurgical surveillance setting. Several studies have explored the role of CTE or CT enteroclysis (9101112) and MRE or MR enteroclysis (131415) in the evaluation of postsurgical CD recurrence, reporting moderate-to-high accuracy for the diagnosis of anastomotic recurrence (79–100% sensitivity and 60–100% specificity). However, other than establishing initial feasibility, these studies are limited in that most of their patients underwent examination more than 12 months post-surgery, and were symptomatic. Therefore, these studies cannot be relied upon for evidence regarding the merits of early postsurgical surveillance.
A determination of the impact of cross-sectional enterography on the early postsurgical surveillance in CD would be clinically meaningful for several reasons. Firstly, patient compliance with the current endoscopic surveillance recommendation, routine ileocolonoscopy 6–12 months after surgery for all postsurgical CD patients, is suboptimal because clinically quiescent patients may refuse to undergo ileocolonoscopy due to its invasiveness (216). Furthermore, although cross-sectional enterography is able to detect clinically unsuspected extraluminal complications of CD (1718), the real magnitude of such benefit is yet obscure in the early postsurgical surveillance setting as its diagnostic yield has not yet been investigated.
Accordingly, we aimed to investigate the diagnostic yield and accuracy of CTE for the early (≤ 12 months) postsurgical surveillance of ileocolic anastomotic recurrence after bowel resection in CD patients.
This observational study was approved by the Institutional Review Board of Asan Medical Center. The requirement for patients' informed consent was waived.
Patients who underwent bowel resection surgery for CD between January 2007 and June 2014 at Asan Medical Center, a tertiary academic center, and who fulfilled the below eligibility criteria, were identified through a search of the institutional inflammatory bowel disease registry. The characteristics of the registry have been previously detailed (19). Briefly, each new patient is registered in a prospective manner, and any relevant data are continuously updated. The study inclusion criteria were age ≥ 18 years at surgery, bowel anastomosis accessible with ileocolonoscopy, and post-surgical follow-up > 1 year. The exclusion criterion was the creation of a bowel stoma, because bowel stomas hinder the standard execution of CTE and ileocolonoscopy. Using these eligibility criteria, 237 consecutive patients were identified. All but eight of these patients had had an ileocolic anastomosis, so these eight were further excluded for a clearer analysis.
Of the 229 patients (Table 1), 88 (60 males and 28 females; mean age ± standard deviation, 31.4 ± 9.6 years at surgery) underwent first-time postsurgical bowel-specific surveillance for recurred/aggravated CD using CTE within 12 months of surgery (3–12 months; median, 6 months). These 88 patients constituted the study cohort. They consisted of 51 patients with a CD activity index (CDAI) < 150 (considered clinically silent) (2) and 37 patients with a CDAI ≥ 150 at the time of CTE. The sections of inflamed bowel were completely removed in the anastomotic region in all patients. The choice of surveillance tests and timing for the postsurgical CD patients was left to the discretion of individual practitioners and the scheduling availability/convenience (i.e., in consideration of waiting list).
Patients were instructed to drink 1200 mL of a 2.5% sorbitol solution over 45–60 minutes, in small portions. CTE was performed using 16-, 32-, or 64-detector row CT scanners (SOMATOM series; Siemens, Erlangen, Germany). After an intravenous injection of a non-ionic iodinated contrast material (120–150 mL of 320–370 mgI/mL) at a rate of 3 mL/sec, enteric-phase images were obtained using the following scan parameters: beam collimation, 16 × 0.75, 32 × 0.6, or 64 × 0.6 mm; beam pitch, 1; gantry rotation time, 0.5 seconds; field of view to fit; 120 kVp; automated tube current modulation (CARE Dose 4D) with quality reference mAs set at 200 mAs; and both axial and coronal images reconstructed with 3-mm thickness at 3-mm intervals.
We used our clinical CTE reading for the primary analysis in this study. Clinical CTE interpretations were performed by experienced, board-certified abdominal radiologists during daily practice, mostly without the endoscopic findings, because the ileocolonoscopy was either requested related to the CTE results or was mostly performed after CTE, as per our institutional practice, if requested concomitantly to CTE as dual-modality surveillance (occasionally used by some practitioners). Since preexisting clinical information can influence clinical reading, and to assess if any bias existed in the original clinical CTE interpretations, an experienced board-certified abdominal radiologist retrospectively re-read all the CTE examinations, having been blinded to the original CTE report and all other clinical information other than the history of bowel surgery for CD. Re-readings were performed at least 1 year after the initial, clinical reading, to prevent recall bias. For both the clinical reading and the re-reading, the presence or absence of recurred CD inflammation was recorded for the anastomotic region (i.e., anastomosis and a short, approximately 10-cm, small bowel proximal to it) and extraluminal penetrating complications were also noted. Positive findings of recurred CD inflammation were according to the established CTE signs of active CD inflammation, including mural thickening (greater than 3 mm), mural hyperenhancement, perienteric stranding/fluid, comb sign, and penetrating complications such as sinus tract, fistula, and abscess.
All medical records, including outpatient clinic visit notes, hospital admission and discharge notes, in-hospital progress notes, emergency department visit notes, surgical operative notes, endoscopy reports, radiology reports, laboratory results, pathology reports, and medications, were thoroughly reviewed. Uneventful cases were routinely followed after the surgery by regular visits to the outpatient clinic, typically at 2-month intervals (with minor deviations at times ranging 1–3 months); along with CDAI measurement; and laboratory tests. To verify the CTE findings and record the CTE-related outcomes, medical record review was performed by a board-certified gastroenterologist and a board-certified abdominal radiologist, both of whom were experienced in the management of CD patients, and uninvolved in other study reading.
Suspected CD recurrence in the anastomotic region was verified by combining all available data, including endoscopic results, other clinical findings and test results, subsequent clinical management decisions, and findings at follow-up evaluations. Endoscopic findings were the major factor for verifying luminal abnormalities. Patients who had indisputable inflammation by CTE often did not undergo endoscopy immediately afterward, but instead underwent interval endoscopic follow-up (within 1 year of CTE in the majority of patients and 1.5 years in most patients). In such cases, CTE findings themselves combined with other compatible clinical data and follow-up endoscopic findings such as residual lesions or healed areas served as verification. Despite some self-referencing in these cases, verification was generally straightforward given the unequivocal CTE findings and the known overall accuracy of CTE (20). Extraluminal penetrating complications were confirmed by surgery, if performed, and by CTE findings, compatible clinical data, and changes during follow-up when patients were medically treated. Findings that could not be determined as true- vs. false-positive findings were categorized as indeterminate.
Step-up therapy that followed a detection of the anastomotic recurrence of CD by CTE was recorded. Stepup therapy was defined as an increased medication dose or addition of immunomodulators or biologics (1).
A subgroup of patients who underwent ileocolonoscopy within one month of CTE were identified to investigate the accuracy of CTE for the diagnosis of CD recurrence in the anastomotic region, using endoscopic Rutgeerts scoring (3) as the reference standard. We limited the analysis to those patients who had an endoscopy within a month of CTE, to achieve a more precise CTE-endoscopy correlation. Patients whose Rutgeerts scores were i0 (no inflammation) or i1 (not more than five aphthous lesions) were considered inactive (1). Scores of i2 (more than 5 aphthous lesions with normal mucosa between the lesions), i3 (diffuse aphthous ileitis), and i4 (diffuse inflammation with already larger ulcers, nodules/cobble, and/or narrowing) were considered to have active inflammation, i.e., anastomotic recurrence (1).
The characteristics of patients who did (n = 88) and did not (n = 141) undergo early CTE surveillance were compared using the Student t test or Fisher exact test, as were those of the patients who did undergo the surveillance. Of this latter group, 51 patients had a CDAI < 150 and 37 had a CDAI ≥ 150. The agreement between the original clinical CTE reading and the blinded re-reading were assessed using the overall proportion of agreement (21).
The diagnostic yield of CTE (the rate of true-positive detection of disease by CTE out of all patients undergoing CTE surveillance) was determined on a per-patient basis for the anastomotic recurrence and extraluminal complications. The proportion of patients who received step-up therapy following a detection of anastomotic recurrence of CD by CTE was determined. These CTE-related outcomes were compared between the two CDAI groups using the Fisher exact test, and a multiple logistic regression analysis to adjust for major known risk factors for CD recurrence such as active smoking, penetrating diseases, and history of another CD-related bowel surgery (17222324).
In the subgroup of patients who underwent ileocolonoscopy within one month of CTE, the sensitivity, specificity, and accuracy of CTE for the anastomotic recurrence were determined. The original reading and the blinded reading were compared using the McNemar test. P < 0.05 was considered statistically significant. Statistical analysis was performed using IBM SPSS Statistics for Windows, version 21.0 (IBM Corp., Armonk, NY, USA).
The characteristics of the 88 patients who underwent CTE surveillance within 12 months after surgery and the 141 patients who did not were not significantly different (Table 1). The characteristics of the 51 patients with CDAI < 150 and 37 patients with CDAI ≥ 150 in the CTE surveillance cohort are summarized in Table 2. Patients with CDAI < 150 showed a slightly younger mean age at surgery and an insignificantly higher smoking rate.
The original clinical CTE interpretation and the blinded re-interpretation showed a 92% (81/88) and a 100% (88/88) agreement for anastomotic recurrence and extraluminal penetrating complications, respectively, indicating that the clinical reading did not have remarkable biases.
According to the original clinical CTE interpretation, CD recurrence was suspected in the anastomotic region of 38 patients, eight of whom also showed extraluminal penetrating complications near the anastomosis site. Of these, one suspected anastomotic recurrence could not be verified (i.e., indeterminate results) and six (15.8% of 38) suspected cases were judged to be false positive. Therefore, the per-patient diagnostic yield of CTE for anastomotic recurrence of CD and extraluminal penetrating complications was 35.2% (31/88) and 9.1% (8/88), respectively. Of the eight patients diagnosed with penetrating complications, two received surgery and six were medically treated. Breakdowns of the CTE yields according to CDAI < 150 (i.e., clinically silent) vs. CDAI ≥ 150 are summarized in Table 3. CTE diagnostic yield for anastomotic recurrence was not significantly different between these two CDAI groups (multivariable adjusted p = 0.623). CTE yield for extraluminal penetrating complications was significantly higher in patients with CDAI ≥ 150 (16.2% [6/37] vs. 3.9% [2/51]; multivariable-adjusted p = 0.029). Exemplary cases are shown in Figures 1, 2, 3.
Step-up therapy occurred following anastomotic recurrence of CD detected by CTE in 20.5% of the patients (18/88). Of these, 17 patients received step-up therapy, and one patient was recommended to have it, but declined. Both were included as per the principle of intention-to-treat principle. The rate was not significantly different between the two CDAI groups (multivariable adjusted p = 0.826) (Table 3). Step-up therapy involved the addition of anti-tumor necrosis factor-α agents (n = 10) and either an increased dose of or the addition of thiopurines (n = 8).
In total, 31 patients (22 males and 9 females; 31 ± 9.5 years at surgery) underwent ileocolonoscopy within one month of CTE (median, 5 days), of whom 13 had recurred active inflammation in the anastomotic region; and 18 did not. The sensitivity and specificity of CTE were 92.3% (12/13) and 83.3% (15/18), respectively, for the original clinical reading, and 84.6% (11/13) and 83.3% (15/18), respectively, for the blinded re-reading (Table 4).
This study suggests that CTE may be a viable option for the early surveillance of recurred CD, including for the evaluation of clinically silent patients, within 12 months of bowel surgery. The diagnostic yields of CTE were fairly high, and CTE presumably played a role in directing step-up therapy in 20.5% of patients. Notably, the CTE yield for anastomotic recurrence was similar between those patients who were clinically silent (CDAI < 150) and those who were not (CDAI ≥ 150). Moreover, the CTE accuracy for the diagnosis of CD recurrence in the anastomotic region in this early surveillance setting was reasonably high. The fact that only 38.4% (88/229) of our eligible postsurgical CD patients underwent early postsurgical CTE surveillance is a research limitation, although this may represent usual clinical practice, particularly given that the recommendation of routine early postsurgical surveillance in CD patients was only recently proposed.
Since, to the best of our knowledge, there are no previous reports of the outcomes of early postsurgical surveillance using CTE (or MRE) in CD patients, our results can only be compared with endoscopic studies. The CTE diagnostic yield shown in this study (35.2% for the anastomotic region) was within and close to the lower margin of the 35–85% rates of 1-year postsurgical ileocolonoscopic CD recurrence reported in randomized controlled trials, and summarized in a systematic review (4). The difference from the endoscopic results could be explained by several factors, including a slightly shorter exact follow-up duration in our study (i.e., CTE performed at a median six postoperative months instead of a full one year follow-up), more thorough data acquisition in controlled trials, and the presumably slightly lower sensitivity of CTE compared with endoscopy. Overall, this comparison suggests CTE as a useful alternative to ileocolonoscopy for the early surveillance of recurred/aggravated CD within 12 months of bowel surgery, when ileocolonoscopy is difficult to perform.
One clear advantage of CTE is its ability to evaluate endoscopically inaccessible areas, particularly the detection of penetrating complications (1718). In this study, CTE detected extraluminal penetrating complications in 9.1% of patients. With endoscopic surveillance, these would either have been diagnosed after a delay, or not at all. Considering that the CTE yield for penetrating complications was significantly greater in patients with CDAI ≥ 150, CTE during the early postsurgical period would likely be more effective for such patients.
The accuracy of CTE for the diagnosis of anastomotic recurrence in these early postsurgical CD patients was deemed acceptable, demonstrating both high sensitivity and high specificity. The CTE findings of anastomotic recurrence are generally not specific except for some advanced cases which show apparent longitudinal ulcers along the mesenteric border in the neo-terminal ileum (Fig. 2). The high specificity of CTE is in part attributed to the fact that other inflammatory conditions that can mimic CD seldom occur in the anastomotic region during the early postsurgical period. Given the rather nonspecific nature of the CTE findings alone, it would be prudent to make a CTE interpretation in association with the clinical setting of each patient, to avoid misdiagnosing any mimicking conditions, even though it is likely to be uncommon.
Although we did not investigate MRE, it may be advantageous for evaluating postsurgical CD patients compared with CTE. MRE does not result in radiation exposure, and provides newer imaging techniques such as diffusion-weighted imaging (252627), which may further assist imaging follow-up of the patients. The impact of MRE specifically for the early postsurgical surveillance in CD remains to be investigated. Given the diagnostic similarity between CTE and MRE (202829), our results may help to make some indirect inferences regarding the role of MRE in early postsurgical surveillance.
This study has several limitations. First, it was a retrospective observational study using clinical data. Unlike randomized trials, some degree of uncertainty and bias regarding the selection of patients who underwent CTE surveillance inevitably exists, although their baseline characteristics were similar to those who did not undergo CTE surveillance. Second, only a portion of the patients had concurrent (within one month) ileocolonoscopy and were eligible for the accuracy analysis. A prospective trial to avoid these limitations and to further confirm the role of cross-sectional enterography for the early postsurgical surveillance in CD may be warranted, given the promising results from this retrospective research. Third, the decision to initiate step-up therapy may not have been solely attributable to a detection of disease recurrence by CTE, but may have also considered other clinical factors.
In conclusion, CTE may be a viable option for early surveillance of recurred disease in CD patients, including the evaluation of clinically silent patients, within 12 months of bowel surgery.
Figures and Tables
Fig. 1
Recurred CD in anastomotic region, i.e., ileum just proximal to ileocolonic anastomosis, seven months after bowel surgery, in 24-year-old woman.
A. CTE shows mild mural thickening (4 mm thickness) and increased mural enhancement in ileum (arrows), just proximal to anastomotic line, with patchy distribution in some areas. Metallic anastomotic suture materials are noted (arrowheads). Unlike neo-terminal ileum, anastomosed colon appears unremarkable. B. Upon endoscopy, multiple small superficial aphthous lesions (arrows), indicative of mild disease, are noted in neo-terminal ileum. CD = Crohn's disease, CTE = CT enterography

Fig. 2
Recurred CD in anastomotic region, i.e., ileum just proximal to ileocolonic anastomosis, five months after bowel surgery, in 18-year-old man.
A. CTE shows deep longitudinal ulcer accompanied by mural thickening and hyperenhancement in mesenteric side of neo-terminal ileum (arrows), which is specific for CD. Prominent vasa recta are also seen. Metallic anastomotic suture materials are noted (arrowheads). B. At endoscopy, large deep longitudinal ulcer (arrows) and swelling of adjacent mucosal folds are noted, which indicate severe inflammation.
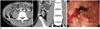
Fig. 3
Recurred CD manifesting as penetrating complication at seven months after bowel surgery in 18-year-old man.
CTE shows 2-cm extraluminal inflammatory lesion (arrow) adjacent to ileocolonic anastomosis (arrowheads), which contains air-bubble to indicate fistulous communication with bowel.
Table 1
Baseline Characteristics of Study Patients Who Underwent and Who Did Not Undergo CTE Surveillance

Table 2
Characteristics of Different Patient Groups According to CDAI in CTE Surveillance Cohort

Table 3
CTE-Related Outcomes According to CDAI Group

Table 4
Diagnostic Performance of CTE for CD Recurrence in Anastomotic Region

References
1. Hashash JG, Regueiro M. A practical approach to preventing postoperative recurrence in Crohn’s disease. Curr Gastroenterol Rep. 2016; 18:25.
2. Yamamoto T. Diagnosis and monitoring of postoperative recurrence in Crohn’s disease. Expert Rev Gastroenterol Hepatol. 2015; 9:55–66.
3. Rutgeerts P, Geboes K, Vantrappen G, Beyls J, Kerremans R, Hiele M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology. 1990; 99:956–963.
4. Buisson A, Chevaux JB, Allen PB, Bommelaer G, Peyrin-Biroulet L. Review article: the natural history of postoperative Crohn’s disease recurrence. Aliment Pharmacol Ther. 2012; 35:625–633.
5. Yang Z, Wu Q, Wu K, Fan D. Management of postoperative Crohn’s disease. Expert Rev Gastroenterol Hepatol. 2014; 8:811–818.
6. Nakase H, Keum B, Ye BD, Park SJ, Koo HS, Eun CS. Treatment of inflammatory bowel disease in Asia: the results of a multinational web-based survey in the 2(nd) Asian Organization of Crohn’s and Colitis (AOCC) meeting in Seoul. Intest Res. 2016; 14:231–239.
7. De Cruz P, Kamm MA, Hamilton AL, Ritchie KJ, Krejany EO, Gorelik A, et al. Crohn’s disease management after intestinal resection: a randomised trial. Lancet. 2015; 385:1406–1417.
8. Burke JP, Doherty GA, O’Connell PR. A survey of current practices used to maintain surgically induced remission following intestinal resection for Crohn’s disease. Int J Colorectal Dis. 2013; 28:1073–1079.
9. Mao R, Gao X, Zhu ZH, Feng ST, Chen BL, He Y, et al. CT enterography in evaluating postoperative recurrence of Crohn’s disease after ileocolic resection: complementary role to endoscopy. Inflamm Bowel Dis. 2013; 19:977–982.
10. Paparo F, Revelli M, Puppo C, Bacigalupo L, Garello I, Garlaschi A, et al. Crohn’s disease recurrence in patients with ileocolic anastomosis: value of computed tomography enterography with water enema. Eur J Radiol. 2013; 82:e434–e440.
11. Soyer P, Boudiaf M, Sirol M, Dray X, Aout M, Duchat F, et al. Suspected anastomotic recurrence of Crohn disease after ileocolic resection: evaluation with CT enteroclysis. Radiology. 2010; 254:755–764.
12. Minordi LM, Vecchioli A, Poloni G, Guidi L, De Vitis I, Bonomo L. Enteroclysis CT and PEG-CT in patients with previous small-bowel surgical resection for Crohn’s disease: CT findings and correlation with endoscopy. Eur Radiol. 2009; 19:2432–2440.
13. Gallego Ojea JC, Echarri Piudo AI, Porta Vila A. [Crohn’s disease: the usefulness of MR enterography in the detection of recurrence after surgery]. Radiologia. 2011; 53:552–559.
14. Koilakou S, Sailer J, Peloschek P, Ferlitsch A, Vogelsang H, Miehsler W, et al. Endoscopy and MR enteroclysis: equivalent tools in predicting clinical recurrence in patients with Crohn’s disease after ileocolic resection. Inflamm Bowel Dis. 2010; 16:198–203.
15. Sailer J, Peloschek P, Reinisch W, Vogelsang H, Turetschek K, Schima W. Anastomotic recurrence of Crohn’s disease after ileocolic resection: comparison of MR enteroclysis with endoscopy. Eur Radiol. 2008; 18:2512–2521.
16. De Cruz P, Bernardi MP, Kamm MA, Allen PB, Prideaux L, Williams J, et al. Postoperative recurrence of Crohn’s disease: impact of endoscopic monitoring and treatment step-up. Colorectal Dis. 2013; 15:187–197.
17. Booya F, Akram S, Fletcher JG, Huprich JE, Johnson CD, Fidler JL, et al. CT enterography and fistulizing Crohn’s disease: clinical benefit and radiographic findings. Abdom Imaging. 2009; 34:467–475.
18. Bruining DH, Siddiki HA, Fletcher JG, Tremaine WJ, Sandborn WJ, Loftus EV Jr. Prevalence of penetrating disease and extraintestinal manifestations of Crohn’s disease detected with CT enterography. Inflamm Bowel Dis. 2008; 14:1701–1706.
19. Park SH, Yang SK, Park SK, Kim JW, Yang DH, Jung KW, et al. Long-term prognosis of Crohn’s disease and its temporal change between 1981 and 2012: a hospital-based cohort study from Korea. Inflamm Bowel Dis. 2014; 20:488–494.
20. Qiu Y, Mao R, Chen BL, Li XH, He Y, Zeng ZR, et al. Systematic review with meta-analysis: magnetic resonance enterography vs. computed tomography enterography for evaluating disease activity in small bowel Crohn’s disease. Aliment Pharmacol Ther. 2014; 40:134–146.
21. Kundel HL, Polansky M. Measurement of observer agreement. Radiology. 2003; 228:303–308.
22. Reese GE, Nanidis T, Borysiewicz C, Yamamoto T, Orchard T, Tekkis PP. The effect of smoking after surgery for Crohn’s disease: a meta-analysis of observational studies. Int J Colorectal Dis. 2008; 23:1213–1221.
23. Simillis C, Yamamoto T, Reese GE, Umegae S, Matsumoto K, Darzi AW, et al. A meta-analysis comparing incidence of recurrence and indication for reoperation after surgery for perforating versus nonperforating Crohn’s disease. Am J Gastroenterol. 2008; 103:196–205.
24. Van Assche G, Dignass A, Reinisch W, van der Woude CJ, Sturm A, De Vos M, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: special situations. J Crohns Colitis. 2010; 4:63–101.
25. Huh J, Kim KJ, Park SH, Park SH, Yang SK, Ye BD, et al. Diffusion-weighted MR enterography to monitor bowel inflammation after medical therapy in Crohn’s disease: a prospective longitudinal study. Korean J Radiol. 2017; 18:162–172.
26. Choi SH, Kim KW, Lee JY, Kim KJ, Park SH. Diffusion-weighted magnetic resonance enterography for evaluating bowel inflammation in Crohn’s disease: a systematic review and meta-analysis. Inflamm Bowel Dis. 2016; 22:669–679.
27. Park SH. DWI at MR enterography for evaluating bowel inflammation in Crohn disease. AJR Am J Roentgenol. 2016; 207:40–48.
28. Fiorino G, Bonifacio C, Peyrin-Biroulet L, Minuti F, Repici A, Spinelli A, et al. Prospective comparison of computed tomography enterography and magnetic resonance enterography for assessment of disease activity and complications in ileocolonic Crohn's disease. Inflamm Bowel Dis. 2011; 17:1073–1080.
29. Lee SS, Kim AY, Yang SK, Chung JW, Kim SY, Park SH, et al. Crohn disease of the small bowel: comparison of CT enterography, MR enterography, and small-bowel follow-through as diagnostic techniques. Radiology. 2009; 251:751–761.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download