Abstract
Objective
The Tubridge flow diverter (FD) is a novel device aimed at reconstructing the parent artery and occluding complex aneurysms. Retreatment of recurrent aneurysms using the FD is challenging. We report our initial experience in the repair of aneurysm recurrence with the FD.
Materials and Methods
A database was reviewed prospectively, and 8 patients with 8 recurrent aneurysms (mean size, 16.7 mm) were identified. Four aneurysms had previously ruptured. The previous aneurysm treatment consisted of coiling in 1 aneurysm and single-stent-assisted coiling in 7 aneurysms. The procedural complications and clinical and angiographic outcomes were analyzed.
Results
Six aneurysms were treated by using a single Tubridge FD alone, while the remaining 2 were treated with FD + coiling. The immediate results of the 8 aneurysms were that they all showed incomplete occlusion. Neither major ischemic nor hemorrhagic complications occurred; however, 1 patient experienced a vasospasm. Follow-up angiographies were available for 7 aneurysms; the mean follow-up was 16.9 months (7–36 months). Five aneurysms were completely occluded, whereas 2 had a residual neck. Severe asymptomatic stenosis of 1 parent artery of a vertebral artery dissecting aneurysm was found. All visible branches covered by the FD were patent. All patients were clinically assessed as having attained a favorable outcome (modified Rankin Scale score ≤ 2) at discharge and follow-up.
Endovascular treatment is now a well-established 859technique for treating intracranial aneurysms (12). However, compared with microsurgery clipping, the durability of endovascular therapy–especially for the wide-neck and/or large one–is considered to be the major limitation (3). Considering the risk of re-rupture (4), it is rational to retreat the recurrent aneurysms. An overall retreatment rate in over 8000 coiled aneurysms was reported to be 10% (5). Although several types of interventions for retreatment are available–including surgical clipping, parent vessel deconstruction, further re-coiling, and/or stent placement (6)–are available, anatomical distortions created by previous coils or stents increase the technical difficulty and risk of complications. Thus, the best retreatment strategy remains unknown.
Flow diversion has emerged as a new strategy to occlude an aneurysm and to reconstruct the parent artery without coils. With the development of material technology, several new flow diverters (FDs) have emerged, and animal experiments have proven their efficacy and safety (789). The Tubridge FD (MicroPort Medical Company, Shanghai, China) is a novel flow diversion device, which disrupts blood flow into the aneurysm sac, inducing thrombosis in the aneurysm sac, while preserving physiological flow in the parent vessel. Preliminary evidence has demonstrated its safety and efficacy for treating complex aneurysms (10). However, data specifically focusing on the use of FD for recurrent aneurysms are limited. The use of FD in these cases has various technical nuances. Therefore, we report our initial experience in the repair of aneurysm recurrence with FD.
After obtaining Institutional Review Board approval, we identified all patients with recurrent aneurysms who were treated with the Tubridge FD at our institution between August 2010 and November 2014 from a prospectively computerized database. Eight patients (4 men, 4 women; median age: 46.5 years) with 8 recurrent aneurysms were identified. Then, their medical charts and images were reviewed. Four patients had a prior history of subarachnoid hemorrhage, and four had a prior history of hypertension. Aneurysm locations were as follows: internal carotid artery (ICA) communicating segment (n = 2), ICA ophthalmic segment (n = 1), ICA cavernous segment (n = 2), and vertebral artery (VA) intradural segment (n = 3). The original mean maximum dimension was 16.7 mm (range: 11.4–34.0 mm).
Previous aneurysm treatment before FD deployment consisted of coiling in 1 aneurysm and single-stent-assisted coiling in 7 aneurysms. Among the stents most commonly used alone were Leo (Balt, Montmorency, France) (n = 3), Enterprise (Cordis Corporation, Miami, FL, USA) (n = 2), Neuroform (Boston Scientific, Natick, MA, USA) (n = 1), and Solitaire (ev3, Irvine, CA, USA) (n = 1). Patient demographics and morphologic features of the aneurysms are summarized in Table 1.
The Tubridge FD is a braided, self-expanding, stent-like device with flared ends (Fig. 1). It is designed with different structures according to various diameters. The small-sized one with a diameter of less than 3.5 mm is composed of 46 nitinol and 2 radio-opaque, platinum-iridium microfilaments (which improves the visualization of both the diameter and the length during the placement procedure). Comparatively, the large-sized one with a diameter greater than or equal to 3.5 mm is composed of 62 nitinol and 2 radio-opaque microfilaments. Thus, the Tubridge FD can offer a relatively higher metal coverage (approximately 30.0 to 35.0 percent), as compared with conventional stents, at the aneurysmal neck after fully opening (11). There is a marker in the middle of the Tubridge FD; the device can still be retracted before being released to that point.
The use of the Tubridge FD was approved by the Ethics Committee of our institution and by the Chinese Food and Drug Administration. For aneurysms with a prior stent, diluted contrast-enhanced (1:1 contrast dilution) Syngo DynaCT (Siemens, Erlangen, Germany) reconstruction was used to detect the apposition of the stent to the wall. The strategy of using FD in recurrent cases was cautiously judged by senior neurointerventionalists to be the best alternative, as measured by both the risk and the potential long-term durability. Written consent was issued by each patient. All patients were pretreated with daily dual antiplatelet drugs consisting of 75 mg clopidogrel (Plavix, Sanofi-Aventis, France) and 300 mg aspirin (Bayasprin, Bayer, Germany) for at least 3 days before the procedure. A prescribed postoperative antiplatelet regimen was administered as follows: 300 mg aspirin + 75 mg clopidogrel within 6 weeks; 100 mg aspirin + 75 mg clopidogrel from 6 weeks to 3 months; and 100 mg aspirin indefinitely after 3 months.
Each surgeon had more than 3 years of experience in intracranial stent placement. If the Syngo DynaCT showed an incomplete wall apposition of the prior stent, pre-dilation with Hyperglide (ev3 Neurovascular, Irvine, CA, USA) balloon was performed to ensure a full opening. The procedures were conducted under general anesthesia. After femoral access was obtained, all patients received systemic heparinization with heparin. Next, a proper (7 Fr) guiding catheter was placed in the distal ICA or VA. For the aneurysms, using Tubridge FD + coiling, coils were delivered through the microcatheter by using mesh technology first, before FD deployment. During the FD implantation, a Tubridge-compatible Endopipe microcatheter (0.029 inch diameter) (MicroPort Medical Company, Shanghai, China) was placed in the distal segment of the aneurysm neck with the assistance of a microwire. Then, the Tubridge FD was introduced through the microcatheter into the target zone. After the FD was placed into position by pushing the delivery wire and simultaneously withdrawing the microcatheter, the device began to expand in the artery and was deployed. Then, diluted contrast-enhanced Syngo DynaCT was used to document the correct expansion of the FD. In the case of incomplete apposition, Hyperglide balloon angioplasty was performed.
According to our follow-up protocol, each patient underwent both a clinical evaluation according to the modified Rankin Scale (mRS) and a digital subtraction angiographic evaluation at 6 months post-treatment and yearly thereafter. All clinical and angiographic follow-up results were recorded and uploaded to the database. We regarded the last data postoperatively as being the final follow-up results. The angiographic results were independently classified by 2 experienced neurointerventionalists according to the 3-point modified Raymond scale (1213). The follow-up angiographic results were also interpreted as three categories, which were compared to reflect changes with the immediate results in the same projections: 1) improvement, defined as decreased contrast filling into the aneurysm sac; 2) stable, defined as unchanged contrast filling into the aneurysm sac; and 3) recurrence, defined as increased contrast filling into the aneurysm sac. Patency of the branches covered by FD, and stenosis (more than 50%) or obliteration of the parent artery, were also both documented.
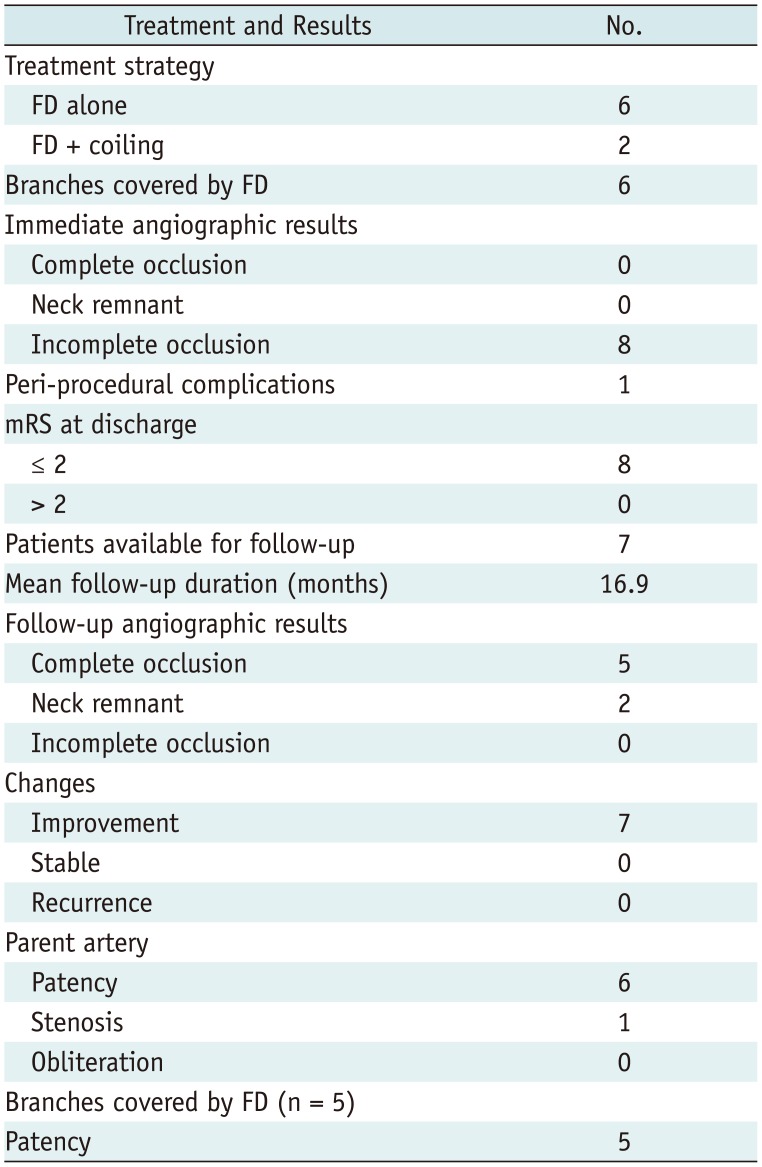
The data of the 8 patients–including the treatment strategy, clinical results, and angiographic results–are presented in Table 2.
Tubridge FD delivery was successful in all 8 patients. Six aneurysms were treated by using a single Tubridge FD alone, while the remaining ones were treated with the Tubridge FD + coiling. After the cautious assessment of the parent arteries and the prior stents, the parent arteries of the selected cases were nearly straight. Therefore, no obvious difficulties with the delivery of the microwire and microcatheter though the prior stents were encountered. All 8 FDs were documented as being good wall appositions, so balloon dilation was not used.
Neither major ischemic nor hemorrhagic complications occurred. except in one patient who experienced a minor procedural complication. This patient had a vasospasm in the ICA and the middle cerebral artery that resolved with 30 mg fasudil (Tianjin Hongri Company, Tianjin, China) infusion intraprocedurally. without neurological defects. Therefore, all patients attained a favorable outcome (mRS ≤ 2) at discharge. Immediate postoperative angiographic images of the 8 aneurysms were all seen as an opacification of the sac. In total, there were 6 visible branches covered by the FD–including 3 ophthalmic arteries, 2 posterior inferior cerebellar arteries, and 1 posterior communicating artery–all of which were patent.
Seven patients received at least one cerebral angiography. Coincidentally, all of these 7 patients were those who had previously received stent-assisted coiling. The last follow-up duration ranged from 7 to 36 months (mean: 16.9 months). All 7 patients maintained an mRS ≤ 2 at each follow-up. Of the 7 aneurysms available for angiographic follow-up, 5 (71.4%) were completely occluded; whereas 2 had a residual neck. So all 7 aneurysms achieved improvement. An illustrated case was shown in Figure 2. During the follow-up period, one parent artery of a VA dissecting aneurysm was found to have severe stenosis without symptoms. Then, a stent angioplasty was performed with an Enterprise on this patient after dilation of a Gateway balloon (Boston Scientific, Natick, MA, USA). This patient twice received angiography follow-up (at 6 months and 13 months post-angioplasty respectively), which showed the VA remained patent without re-stenosis. The remaining 6 parent arteries were patent. Six visible branches, in 6 cases, were covered by the FD during the procedure. One patient with 1 ophthalmic artery covered by the FD did not have a follow-up angiographic evaluation because of loss to follow-up. Five branches, whose follow-up angiographic results were available, were patent without obliteration.
Our initial experience related to the application of the Tubridge FD in recurrent aneurysms showed satisfactory results in selected patients with a high rate of complete occlusion at follow-up. Although 7 parent arteries each had 1 conventional stent implanted before the deployment of the FD, the Tubridge FD had no obvious influence on the covered side branches (including the small posterior inferior cerebellar arteries).
Endovascular interventions for both ruptured and unruptured intracranial aneurysms have come to the forefront since the publications of the International Subarachnoid Aneurysm Trial (2414). However, these studies also clearly demonstrated that the risk of recurrence was substantially higher in coil embolism aneurysms (1415). Numerous studies have proven that a larger size and a ruptured status were significant risk factors implicated in recurrence (16171819). Following the coiling of large and giant aneurysms, recurrence rates ranged from 37.5% to 90%, approximately. Although the stent assisted-coiling technique has shown improved durability, it still remains unsatisfactory. In a series of 334 large and giant aneurysms, recurrence rates were 44% for unassisted coiling and 32.5% for stent-assisted coiling (20). In our report, all 8 recurrent aneurysms were either large or giant aneurysms (original maximum dimension ≥ 10 mm)–and half of them had previously ruptured. In accordance with the prior data, these aneurysms had a high risk of recurrence, even though 7 aneurysms had previously been treated with a prior stent.
It is conceivable that only by achieving definitive and durable exclusion of the aneurysms can preventively protect patients vulnerable to an aneurysm rupture. Whether coil embolization or surgical clipping had been performed, the Cerebral Aneurysm Rerupture After Treatment study (CARAT) found that a 1.1% risk of rebleeding was observed in conjunction with complete occlusion, a 5.9% risk was seen for a 70–90% occlusion, and a 17.6% risk was found for a less-than-70% occlusion. Additionally, no rebleeding was noted after retreatment procedures in the CARAT study (21). Consequently, retreatment of recurrent aneurysms is rational. Several series, each with large numbers of conventional endovascular retreatments, showed that the overall morbidity and mortality ranged from 1.3% to 3% (222324)–thus demonstrating the safety of re-treating recurrent aneurysms by using endovascular approaches. However, its the retreatment's efficacy was uncertain. In a study of long-term outcomes (19), 48.6% of recoiled (coiling alone) aneurysms showed a second recurrence at a mean follow-up of 15.5 ± 18.4 months. After stent-assisted embolization of recurrent aneurysms, 16% still needed additional endovascular treatment because of coil compaction and regrowth of the recurrent aneurysm (25). Therefore, novel techniques should be attempted to improve the outcomes of endovascular retreatment.
Endoluminal reconstruction of the parent artery with FD represents a novel and promising technique. Our study found that 71.4% of the aneurysms available for angiographic follow-up achieved complete occlusion, which suggested the efficacy of the retreatment with the FD. However, due to a chance of late recurrence of large aneurysms treated by FD (26), a follow-up of more than 2 years is still required for these cases. In our case series, 7 of 8 aneurysms had previously received single-stent-assisted coiling. In accordance with our results, Fischer et al. (27) reported that a series of re-treatment lesions using Pipeline embolization device (PED) showed that the occlusion rates at 6 months in cases within a prior stent were similar to those without a prior stent (65 and 69%, respectively). Chalouhi et al. (28) reported that 64.3% of 14 patients with angiographic follow-up had complete aneurysm occlusion; however, they believed that a previous stent in situ may result in incomplete occlusion. In aneurysms which were recanalized after stent-assisted embolization, Heiferman et al. (29) found that 76% showed improved Raymond class occlusion at the 12-month follow-up, with 38% being completely occluded. The study–focusing on the pipeline device in the treatment of recurrent, previously stented aneurysms–showed complete aneurysm occlusion in 55.6% of 21 previously stented aneurysms (30). In agreement with those studies, we also think that management of recurrent previously stented aneurysms is a therapeutic challenge. A previously deployed stent may cause poor vessel wall apposition of the FD, resulting in an endoleak between the FD and the vessel wall. This might be the reason for persistent aneurysm filling. Therefore, in our study, each case was carefully selected based on the evaluation of the target vessel and wall apposition of the previous stent before the subsequent procedure. A selection bias caused by the small size of our study may have affected the results.
In our study, the rate of procedure-related complication was acceptable. The vasospasm was not a specific complication of the FD. Fortunately, an asymptomatic stenosis was detected at routine follow-up. After the deployment of an Enterprise stent following a balloon angioplasty, the parent artery did not develop re-stenosis. An assessment of complications in the recoiling of recurrent aneurysms had reported that the procedural permanent morbidity was 1.8%. In the series of stent-assisted recoiling of recurrent aneurysms (31), the technical success rate was 91% and the procedural complications rate was 11% (31). In a report of 15 recurrent aneurysms retreated with the PED, major complications occurred in 1 patient and minor complications in 4 patients (28). Heiferman et al. (29) documented 3 adverse events in a series of 25 patients. The reported complications included intraparenchymal hemorrhage, in-stent thrombosis, carotid-cavernous fistula, and vessel dissection. Most of the complications were not related to the use of FD in the retreatment itself. Early in-stent thrombosis and subsequent parent artery occlusion were always related to poor FD opening. For patients with full opening of the FD, the rate of ischemic event was reported to be nothigher than that for the self-expanding stent (323334). A poor FD opening may be caused by several technical problems including mistakes in the microguidewire's crossing of the stent through the cells; microcatheter access failure; FD “catching” on the prior stent struts; and an inappropriate landing zone.
It should be noted that the use of Tubridge FD in recurrent, previously stented aneurysms is only an alternative option for selected patients owing to its technical difficulties and potential complications. We believe that attention should be paid to several technical points when using this method. First, it may be helpful to ensure the full expansion of the previous stent without kinking in certain cases by using Syngo DynaCT reconstruction. Second, cautious assessments of the route and target vessel before the procedure are of vital importance. A straight shape, with an equal diameter or at least a gradual diameter change of the target vessel create conditions for a good wall apposition. Target vessels with a stenosis or a tortuous curve should be excluded. Third, when a microguidewire is advanced through the previous stent (especially the open-cell stent), the rapid rotation and pushing a J-shaped-tip microguidewire is an effective technique. It will prevent microguidewire entry through the cells of previous stents. Moreover, when there is uncertainty about the status of the microguidewire, a balloon could be advanced through the microguidewire and dilated, in order to confirm the position of the microguidewire by discriminating it from the morphology of the balloon. Fourthly, during deployment, the distal landing point of the FD should be positioned accurately, and the FD should not be withdrawn to avoid the migration of the prior stent during releasing. Finally, before and after FD deployment, angioplasty with a compliant balloon may be necessary to obtain the best apposition of the implant to the vessel wall.
This study has some major limitations, including the small series size, the short angiographic follow-up period of some cases, retrospective design, single-center nature, and lack of randomized comparisons with other potentially efficacious therapies.
In selected patients, the Tubridge FD can provide a safe and efficient option for the retreatment of recurrent aneurysms. Nevertheless, attention should be paid to several technical points.
References
1. McDonald JS, McDonald RJ, Fan J, Kallmes DF, Lanzino G, Cloft HJ. Comparative effectiveness of unruptured cerebral aneurysm therapies: propensity score analysis of clipping versus coiling. Stroke. 2013; 44:988–994. PMID: 23449260.
2. Lin N, Cahill KS, Frerichs KU, Friedlander RM, Claus EB. Treatment of ruptured and unruptured cerebral aneurysms in the USA: a paradigm shift. J Neurointerv Surg. 2012; 4:182–189. PMID: 21990481.

3. Campi A, Ramzi N, Molyneux AJ, Summers PE, Kerr RS, Sneade M, et al. Retreatment of ruptured cerebral aneurysms in patients randomized by coiling or clipping in the International Subarachnoid Aneurysm Trial (ISAT). Stroke. 2007; 38:1538–1544. PMID: 17395870.

4. Molyneux A, Kerr R, Stratton I, Sandercock P, Clarke M, Shrimpton J, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised trial. Lancet. 2002; 360:1267–1274. PMID: 12414200.

5. Ferns SP, Sprengers ME, van Rooij WJ, Rinkel GJ, van Rijn JC, Bipat S, et al. Coiling of intracranial aneurysms: a systematic review on initial occlusion and reopening and retreatment rates. Stroke. 2009; 40:e523–e529. PMID: 19520984.
6. Dorfer C, Gruber A, Standhardt H, Bavinzski G, Knosp E. Management of residual and recurrent aneurysms after initial endovascular treatment. Neurosurgery. 2012; 70:537–553. discussion 553-554. PMID: 21904266.

7. Kim BM, Kim DJ, Kim DI. A new flow-diverter (the FloWise): in-vivo evaluation in an elastase-induced rabbit aneurysm model. Korean J Radiol. 2016; 17:151–158. PMID: 26798228.

8. Huang QH, Yang PF, Zhang X, Shi Y, Shao XM, Liu JM. [Effects of flow diverter with low porosity on cerebral aneurysms: a numerical stimulative study]. Zhonghua Yi Xue Za Zhi. 2010; 90:1024–1027. PMID: 20646519.
9. Simgen A, Ley D, Roth C, Yilmaz U, Körner H, Mühl-Benninghaus R, et al. Evaluation of a newly designed flow diverter for the treatment of intracranial aneurysms in an elastase-induced aneurysm model, in New Zealand white rabbits. Neuroradiology. 2014; 56:129–137. PMID: 24496551.

10. Zhou Y, Yang PF, Fang YB, Xu Y, Hong B, Zhao WY, et al. A novel flow-diverting device (Tubridge) for the treatment of 28 large or giant intracranial aneurysms: a single-center experience. AJNR Am J Neuroradiol. 2014; 35:2326–2333. PMID: 24722307.

11. Hong B, Wang K, Huang Q, Xu Y, Fang X, Li Z, et al. Effects of metal coverage rate of flow diversion device on neointimal growth at side branch ostium and stented artery: an animal experiment in rabbit abdominal aorta. Neuroradiology. 2012; 54:849–855. PMID: 22170078.

12. Suh SH, Cloft HJ, Lanzino G, Woodward K, Kallmes DF. Interobserver agreement after pipeline embolization device implantation. AJNR Am J Neuroradiol. 2013; 34:1215–1218. PMID: 23275597.

13. Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke. 2001; 32:1998–2004. PMID: 11546888.

14. Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005; 366:809–817.

15. Darsaut TE, Raymond J. Barrow Ruptured Aneurysm Trial: 3-year results. J Neurosurg. 2013; 119:1642–1644.
16. Taki W, Sakai N, Suzuki H. Prospective Registry of Subarachnoid Aneurysms Treatment (PRESAT) group. Importance of independent evaluation of initial anatomic results after endovascular coiling for ruptured cerebral aneurysms. J Clin Neurosci. 2013; 20:527–531. PMID: 23324438.

17. Taki W. PRESAT group. Sakai N, Suzuki H. Factors predicting retreatment and residual aneurysms at 1 year after endovascular coiling for ruptured cerebral aneurysms: Prospective Registry of Subarachnoid Aneurysms Treatment (PRESAT) in Japan. Neuroradiology. 2012; 54:597–606. PMID: 21861080.
18. Nguyen TN, Hoh BL, Amin-Hanjani S, Pryor JC, Ogilvy CS. Comparison of ruptured vs unruptured aneurysms in recanalization after coil embolization. Surg Neurol. 2007; 68:19–23. PMID: 17586214.

19. Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003; 34:1398–1403. PMID: 12775880.

20. Chalouhi N, Tjoumakaris S, Gonzalez LF, Dumont AS, Starke RM, Hasan D, et al. Coiling of large and giant aneurysms: complications and long-term results of 334 cases. AJNR Am J Neuroradiol. 2014; 35:546–552. PMID: 23945229.

21. Johnston SC, Dowd CF, Higashida RT, Lawton MT, Duckwiler GR, Gress DR. CARAT Investigators. Predictors of rehemorrhage after treatment of ruptured intracranial aneurysms: the Cerebral Aneurysm Rerupture After Treatment (CARAT) study. Stroke. 2008; 39:120–125. PMID: 18048860.
22. Ringer AJ, Rodriguez-Mercado R, Veznedaroglu E, Levy EI, Hanel RA, Mericle RA, et al. Defining the risk of retreatment for aneurysm recurrence or residual after initial treatment by endovascular coiling: a multicenter study. Neurosurgery. 2009; 65:311–315. discussion 315. PMID: 19625910.
23. Renowden SA, Koumellis P, Benes V, Mukonoweshuro W, Molyneux AJ, McConachie NS. Retreatment of previously embolized cerebral aneurysms: the risk of further coil embolization does not negate the advantage of the initial embolization. AJNR Am J Neuroradiol. 2008; 29:1401–1404. PMID: 18436614.

24. Henkes H, Fischer S, Liebig T, Weber W, Reinartz J, Miloslavski E, et al. Repeated endovascular coil occlusion in 350 of 2759 intracranial aneurysms: safety and effectiveness aspects. Neurosurgery. 2008; 62(6 Suppl 3):1532–1537. PMID: 18695570.

25. Tähtinen OI, Manninen HI, Vanninen RL, Rautio R, Haapanen A, Seppänen J, et al. Stent-assisted embolization of recurrent or residual intracranial aneurysms. Neuroradiology. 2013; 55:1221–1231. PMID: 23861213.

26. Zhang X, Lv N, Wang C, Cao W, Liu J, Huang Q. Late recurrence of a completely occluded large intracranial aneurysm treated with a Tubridge flow diverter. J Neurointerv Surg. 2017; 9:e6. PMID: 27342762.

27. Fischer S, Vajda Z, Aguilar Perez M, Schmid E, Hopf N, Bäzner H, et al. Pipeline embolization device (PED) for neurovascular reconstruction: initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology. 2012; 54:369–382. PMID: 21881914.

28. Chalouhi N, Chitale R, Starke RM, Jabbour P, Tjoumakaris S, Dumont AS, et al. Treatment of recurrent intracranial aneurysms with the pipeline embolization device. J Neurointerv Surg. 2014; 6:19–23. PMID: 23345630.

29. Heiferman DM, Billingsley JT, Kasliwal MK, Johnson AK, Keigher KM, Frudit ME, et al. Use of flow-diverting stents as salvage treatment following failed stent-assisted embolization of intracranial aneurysms. J Neurointerv Surg. 2016; 8:692–695. PMID: 26041098.

30. Daou B, Starke RM, Chalouhi N, Tjoumakaris S, Hasan D, Khoury J, et al. Pipeline embolization device in the treatment of recurrent previously stented ccerebral aneurysms. AJNR Am J Neuroradiol. 2016; 37:849–855. PMID: 26611991.
31. Sedat J, Chau Y, Moubarak K, Vargas J, Lonjon M. Endovascular treatment of recurrent coiled aneurysms: assessment of complications and rebleeding during a decade in a single center. Interv Neuroradiol. 2012; 18:14–19. PMID: 22440596.

32. Wagner A, Cortsen M, Hauerberg J, Romner B, Wagner MP. Treatment of intracranial aneurysms. Reconstruction of the parent artery with flow-diverting (Silk) stent. Neuroradiology. 2012; 54:709–718. PMID: 21894512.

33. Velioglu M, Kizilkilic O, Selcuk H, Kocak B, Tureci E, Islak C, et al. Early and midterm results of complex cerebral aneurysms treated with silk stent. Neuroradiology. 2012; 54:1355–1365. PMID: 22695740.

34. Yu SC, Kwok CK, Cheng PW, Chan KY, Lau SS, Lui WM, et al. Intracranial aneurysms: midterm outcome of pipeline embolization device--a prospective study in 143 patients with 178 aneurysms. Radiology. 2012; 265:893–901. PMID: 22996749.

Fig. 1
Tubridge flow diverter is new type of flow diversion device developed by MicroPort Medical Company.
It was designed with pore size of 0.040–0.050 mm2 at nominal diameter, aiming to provide high metal coverage (approximately 30.0 to 35.0 percent).
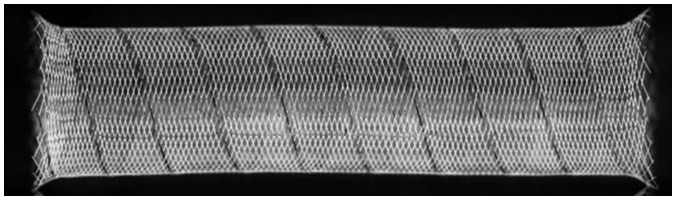
Fig. 2
An illustrated recurrent aneurysms treated by flow diverter.
A. Left internal carotid artery three-dimensional reconstruction showing large ophthalmic segment aneurysm of approximately 20.4 mm. B. aneurysm was treated with Neuroform stent-assisted coiling, and immediate result showed residual sac. C. At 33-month follow-up, aneurysm was significantly recurrent (arrowhead). D, E. Cross-sectional images by DynaCT showing markers and morphology of prior stent (white arrows). F. Unsubtracted working projection showing FD implantation procedure. Three white arrows point to distal end, proximal end, and marker in middle of FD, respectively. G. Postoperative angiography revealing decreased filling of sac. And ophthalmic artery covered by FD was patent (black arrow). H. 11-month follow-up angiography revealing that aneurysm is completely occluded with ophthalmic artery being patent (arrowhead). FD = flow diverter
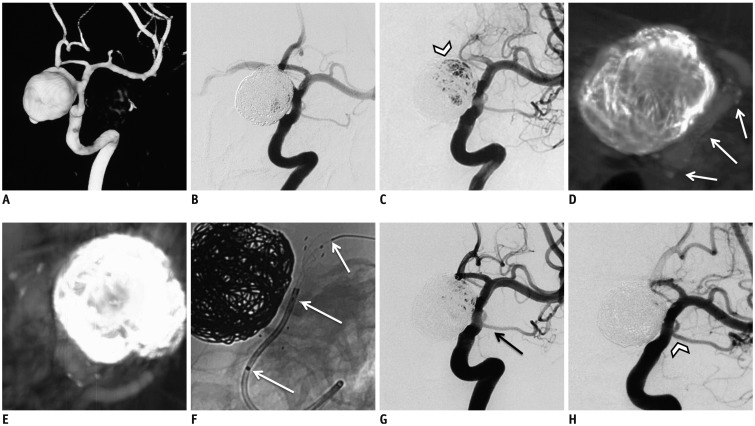
Table 1
Demographics of Patients and Morphologic Features of Aneurysms
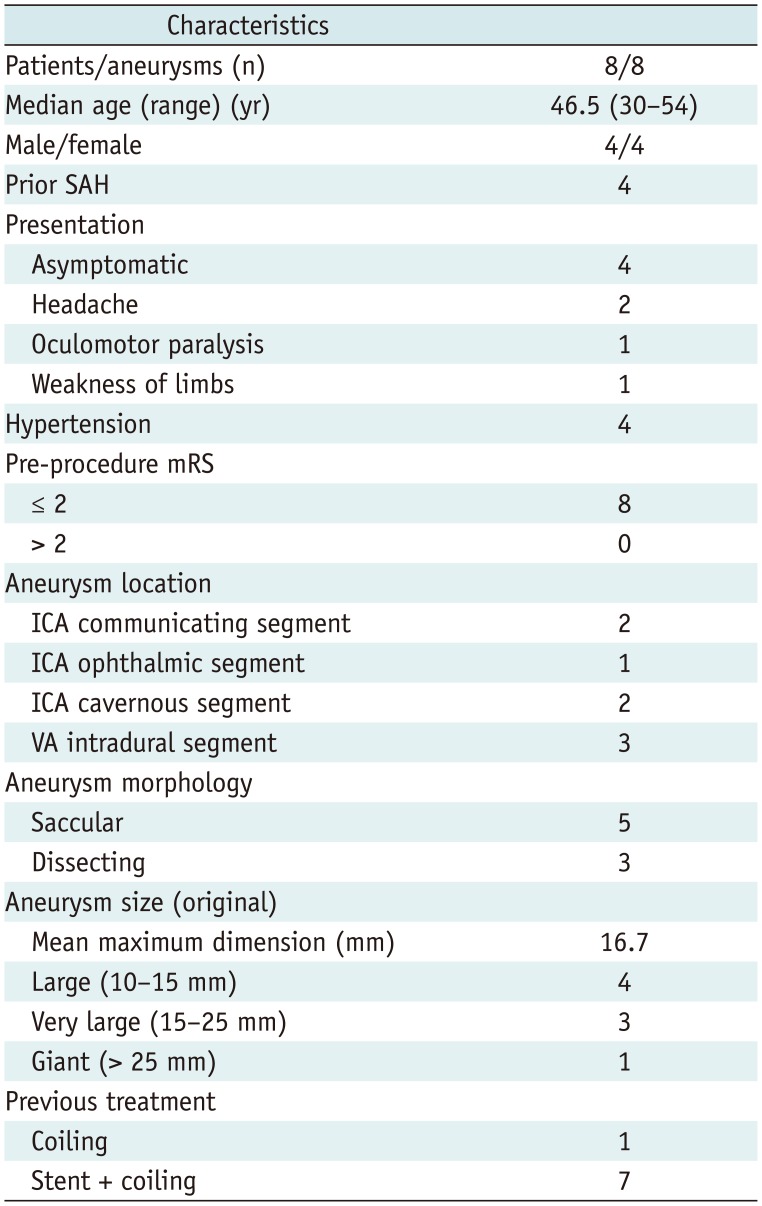
Table 2
Treatment, Clinical, Angiographic, and Follow-Up Results




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download