Abstract
The management of gastrointestinal stromal tumors (GISTs) has evolved significantly in the last two decades due to better understanding of their biologic behavior as well as development of molecular targeted therapies. GISTs with exon 11 mutation respond to imatinib whereas GISTs with exon 9 or succinate dehydrogenase subunit mutations do not. Risk stratification models have enabled stratifying GISTs according to risk of recurrence and choosing patients who may benefit from adjuvant therapy. Assessing response to targeted therapies in GIST using conventional response criteria has several potential pitfalls leading to search for alternate response criteria based on changes in tumor attenuation, volume, metabolic and functional parameters. Surveillance of patients with GIST in the adjuvant setting is important for timely detection of recurrences.
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors of the gastrointestinal tract. Though initially labeled histologically as leiomyosarcomas, the identification of unique activating mutations in KIT gene enabled classification of GIST as a distinct entity (1). GIST like other soft tissue sarcomas was conventionally associated with poor prognosis with a 5-year survival of 5–20% mainly due to resistance to conventional chemotherapy and radiotherapy (23). The discovery of imatinib mesylate which is a small molecule inhibitor of receptor tyrosine kinases (RTK) notably KIT dramatically changed the outcome of patients with GIST (4). In the last one and half decade following the discovery of KIT and imatinib, several new mutations responsible for the pathogenesis of GIST have been discovered. Concurrently several tyrosine kinase inhibitors (TKIs) many of them effective in GIST have also been discovered and approved by the United States Food and Drug Administration (4). In addition, better understanding of the biologic behavior of GIST led to proposition of several risk prediction models to streamline management and surveillance strategies. In this manuscript, we will provide an up to date review of the current concepts on mutational taxonomy, risk stratification, targeted therapies and surveillance strategies in patients with GIST emphasizing the role of radiologists.
Gastrointestinal stromal tumors characteristically express a type III RTK, KIT in more than 90% of cases which is used for characterizing them on immunohistochemistry (5). In 80–85% of GISTs, the KIT gene is mutated which leads to non-ligand dependent autonomous activation of down-stream signal pathways, a key step in the pathogenesis in GIST (6). In another 5–10% cases of GIST, a mutation in the platelet derived growth factor receptor alpha (PDGFRA) gene encoding another similar RTK drives the pathogenesis of GIST (6). Several types of mutations have been discovered in KIT and PDGFRA and vary from point mutations to frame-shift insertions or deletions. Mutations in KIT can involve exon 9, 11, 13, and 17 (7). The most common of these are mutations in exon 11 occurring in greater than 60% cases, often in gastric GISTs (Fig. 1) (8). Exon 9 mutations on the other hand tend to occur in small bowel GISTs (Fig. 2) (8). Primary mutations in exon 13 and 17 are rare but can occur as secondary mutations as a mechanism of resistance (9). Mutations in PDGFRA include exon 14, 12, and 18 (9). Majority of PDGFRA mutant GISTs are gastric in origin.
In up to 10–15% cases of GIST, both KIT and PDGFRA genes carry wild type sequences (4). The pathogenesis of such wild-type GISTs is an active area of research. While some of these tend to be sporadic in origin, some are associated with clinical syndromes like neurofibromatosis, Carney-Stratakis syndrome and Carney triad. Recent studies have shown that some of the erstwhile wild-type GISTs have non-KIT and non-PDGFRA driver mutations like BRAF V600E and succinate dehydrogenase (SDH) gene mutations (10).
Among the previously wild-type GISTs, GISTs with mutations in SDH subunits have garnered interest due to unique epidemiologic, clinical and histopathologic features–increased incidence of young, female patients, association with paragangliomas, gastric origin, epithelioid or mixed epithelioid and spindle cell type and lymphatic spread at histopathology (Fig. 3) (11). SDH is a key enzyme in the mitochondrial citric acid pathway which has several subunits (A, B, C, and D). Loss of function of SDH is seen in patient with Carney-Stratakis syndrome, Carney triad, familial paraganglioma syndromes and pediatric GISTs (11).
The biologic behavior of GIST tends to be determined by the mutational status. Mutations of KIT exon 11, PDGFRA and SDH subunits are often gastric in origin where as GISTs with KIT exon 9 and neurofibromatosis are often small bowel in origin (12). SDH-deficient GISTs tend to be multifocal and frequently metastasize to nodes in addition to liver and peritoneum (13). In addition, presence of extraadrenal paragangliomas and/or pulmonary chondromas in patients with GIST can hint towards SDH-deficient GIST in the setting of Carney-Stratakis syndrome or Carney triad (11).
The type of mutation in GIST also predicts the risk of recurrence and long-term outcome in GIST. Deletions in exon 11 are associated with aggressive behavior where as point mutations in exon 11 portend a better prognosis (7). Small bowel GISTs with exon 9 mutations are aggressive (Fig. 2) (7). PDGFRA mutant GISTs in general has an indolent course (7). Similarly, patients with SDH-deficient GISTs have an indolent course in spite of metastasis (Fig. 3) (13). The type of mutation also determines response to imatinib (141516). Exon 11 mutant GISTs respond dramatically to imatinib (Fig. 1). Exon 9 mutant GISTs, SDH-deficient GISTs and wild-type GISTS are inherently resistant to treatment with imatinib (Fig. 3) (17). This type of resistance is usually encountered in the first 6 months of therapy and referred to as primary resistance (151618). Exon 11 mutants can develop resistance after initial response to imatinib usually after 6 months referred to as secondary resistance (17). Secondary resistance in exon 11 GISTs usually occurs due to secondary mutations in exons 13 and 17 (7). Upfront knowledge of the type of mutation can help radiologists in prompt interpretation of primary resistance to treatment with imatinib.
Gastrointestinal stromal tumors have a complex biologic behavior which makes predicting their malignant potential difficult. Virtually all GISTs irrespective of size are considered malignant. As such, efforts have been made over the years to design consensus criteria, which can enable stratifying GISTs according to risk of recurrence or metastasis. The National Institute of Health (NIH) consensus criteria proposed in 2001 were based on two important features: mitotic count and tumor size (Fig. 4) (19). Subsequently Miettinen and Lasota (20) in a large series of GISTs arising from various sites of gastrointestinal tract found that in addition to tumor size and mitotic count, the site of origin also determined the risk of recurrence in GIST and proposed the Armed Forces Institute of Pathology (AFIP) risk criteria in 2006 (Fig. 4). In 2008, Joensuu (21) proposed the modified NIH consensus criteria taking tumor site and tumor rupture into account as tumor rupture either spontaneously or during surgery significantly increases recurrence risk (22). Few other modifications were also proposed by other authors for example Goh et al. (23) and Huang et al. (24).
The Memorial Sloan Kettering Cancer Center (MSKCC) nomogram was developed in 2009 from 127 patients with GIST to accurately predict individual recurrence-free survival (RFS) after resection of localized GIST and validated in two other cohorts (25). The nomogram was based on points assigned in a continuous non-linear fashion for tumor size, site and mitotic count and the total number of points then used to determine the 2- and 5-year RFS. The nomogram had high predictive value for 5-year RFS than NIH criteria or the AFIP criteria. The inclusion of mutational status did not alter the accuracy of the nomogram. Another study designed to validate the MSKCC nomogram and compare it with the NIH, AFIP and Joensuu criteria found that the MSKCC nomogram and the AFIP criteria performed better than the other two criteria in estimating the RFS (26). The reason for the better performance of MSKCC nomogram and AFIP criteria was hypothesized to be related to the greater emphasis on tumor site in these criteria compared to NIH or Joensuu criteria (26).
In a pooled population-based cohort of 2560 patients with operable GIST who did not receive adjuvant imatinib, an attempt was made to refine the existing risk stratification criteria (27). In this study, large tumor size, high mitotic count, non-gastric location, tumor rupture and male sex were found to have independent prognostic significance. While NIH, AFIP, and Joensuu criteria all predicted outcome accurately, the Joensuu criteria was the best criteria to identify patients with highest risk of recurrence (27). The authors in this study also generated novel heat contour maps using tumor size and mitotic count as continuous non-linear variables along with tumor site and tumor rupture. These maps provided individualized patient outcomes and were more accurate than the existing criteria in estimating recurrence risk implying that treating mitotic count and tumor size as non-linear continuous variables is better than categorizing them (27). Though mutational status affects the risk of recurrence, this information was available in very few patients and was not incorporated in analysis (27).
Several other factors like tumor necrosis, vascularity, invasion of adjacent viscera have been shown to increase risk of recurrence in GIST (7). Further refining of the risk stratification schemes using these additional factors can increase the accuracy of prediction models. Recently there has been increase in interest in identifying imaging biomarkers which can predict long-term outcome in GIST patients. Both tumor size and mitotic count at histopathology can be subject to variations across institutions. Furthermore, variability in expertise of subjective assessment of mitotic count, alteration of mitotic count following neoadjuvant imatinib therapy and possibility of non-representative biopsy samples due to tumor heterogeneity can be challenges which can be difficult to address (28). Accordingly, predicting risk based on pre-operative imaging features can be alluring.
In a study of 143 patients with gastric GIST at our institute, pre-operative treatment naïve CT morphologic features were predictive of metastasis in GIST (28). On multivariate analysis, tumor size > 10 cm, irregular lobulated outline and enhancing solid component were independent predictors of metastasis (Fig. 5) (28). These features were also associated with higher mitotic count and poor overall outcome. In tumors 10 cm or smaller in size, enhancing solid component and irregular/lobulated outline were associated with metastasis (28). While these CT imaging features can be anticipated to be incorporated in the risk stratification schemes in the future, they can help in management decisions in some patients who receive neoadjuvant therapy and closer surveillance of patients with small GISTs with enhancing component or irregular/lobulated outline (28). Larger studies will be required to design imaging-based risk stratification model. The role of volumetric analysis in risk stratification is yet to be studied.
Surgery is the treatment of choice for all resectable GISTs. However, targeted therapy with imatinib and other TKIs is now widely used in the management of GIST in various settings. Neoadjuvant imatinib is used preoperatively to downsize the tumor to enable less morbid organ-sparing surgeries (29). After successful resection of GIST, imatinib is administered in the adjuvant setting to prolong the progression free survival (PFS) (30). Currently the duration of adjuvant imatinib is three years, although there are ongoing trials evaluating the advantage of 5-year adjuvant imatinib (3031). In the metastatic setting, imatinib is the first-line of treatment (32). In patients demonstrating primary or secondary resistance to imatinib, second-line sunitinib is the treatment of choice (33). Resistance to sunitinib is currently managed with third-line regorafenib (34). In patients who are refractory to all lines of treatment, a recent study has shown that rechallenge with imatinib can slow the disease progression as some of the tumor clones tend to remain sensitive to imatinib (35).
Computed tomography (CT) is routinely used in monitoring the response to treatment in GIST. Both primary and metastatic GIST have a unique morphologic response to imatinib on CT scan (Figs. 1, 5) (36). The heterogeneously enhancing primary and metastatic lesions in GIST tend to show dramatic decrease in the enhancing component after treatment with little or no change in lesion size (Fig. 5). A transient increase in size can actually be seen in some lesions. This atypical pattern of treatment response causes ambiguity when change in size according to Response Evaluation Criteria In Solid Tumors (RECIST) is used for interpreting response. Accordingly, alternate tumor response criteria incorporating changes in tumor attenuation along with size reduction were proposed by Choi et al. (37). According to the Choi criteria, a 15% decrease in CT attenuation or 10% decrease in unidimensional size indicates response in contrast to 30% decrease in unidimensional size as per RECIST. In a study of 40 patients with GIST treated with imatinib and evaluated with pre- and post-treatment CT and 18F-fluorodeoxy glucose (FDG) positron emission tomography (PET)/CT, Choi criteria had greater sensitivity in identifying responders compared to RECIST although both had similar specificity and correlated better with disease-specific survival (37).
Given that both primary and metastatic tumors in GIST tend to be irregular rather than spherical (as assumed by RECIST), it is often argued that volume is a better representation of the actual number of tumor cells than single longest diameter (38). Accordingly, moderate changes in size can be detected better using volumetric analysis rather than RECIST (39). The utility of volumetric analysis in assessing response to treatment in hepatic metastasis from GIST was attempted by Schiavon et al. (40) in two independent studies consisting of 84 and 78 patients with hepatic metastases from GIST who were treated with imatinib (41). While both Choi criteria and volumetric criteria identified more number of imatinib responders compared to RECIST, only volumetric criteria had better correlation with overall survival (4041). They concluded that GIST metastasis should be conceptualized mathematically as ellipsoidal lesions rather than spheroidal (4041). Similarly, in the case of primary GISTs, in a study of 127 patients at our institute, we found that the actual tumor volume in primary GISTs can be replicated using the mathematical model for scalene ellipsoid which relies on three measured axes compared to single axis in spheroids (39).
Patients who fail to respond or develop resistance to imatinib are treated with second- and third-line TKIs. It is not known if the dramatic density changes seen with imatinib occur when challenged with new TKIs. Few recent studies have attempted to study the various treatment response criteria in metastatic GIST patients treated with second- and third-line TKIs and correlate them with survival (424344). In the study by Schramm et al. (42), RECIST, Choi and volumetric criteria were compared in 20 patients with metastatic GIST treated with second-line sunitinib at 3-months and 1-year intervals after start of treatment and were correlated with disease specific survival (DSS). The authors found that though Choi criteria classified more number of patients as partial responders on 3-month and 1-year scans, partial responders by Choi criteria at 1-year follow-up had shorter DSS than patients with stable disease (SD) or progressive disease (PD) (42). The best correlation of DSS was with RECIST. Partial responders as per RECIST had the longest DSS and patients with PD per RECIST had the shortest survival on both 3-month and 1-year scans (42). Accordingly, the authors concluded that Choi criteria may not be helpful in identifying patients who tend to have longer survival on follow-up scans while treating with second-line sunitinib (42).
In another study performed at our institute, Shinagare et al. (44) compared Choi criteria, RECIST, RECIST 1.1 and World Health Organization (WHO) criteria in 20 patients with advanced GIST treated with third-line regorafenib in a phase II trial. Similar to other studies, Choi criteria identified more number of patients as partial responders. However, clinical benefit rate defined as complete or partial response or SD for ≥ 16 weeks were similar among all tumor response criteria (44). The PFS was longest for RECIST 1.1 and shortest for Choi criteria. Furthermore, the PFS was strongly concordant with overall survival by RECIST, RECIST 1.1 and WHO criteria but not by Choi criteria (44). The authors therefore concluded that in the current scenario using RECIST 1.1 based response evaluation may be prudent especially in clinical trial setting as Choi criteria due to high sensitivity would progress patients sooner than RECIST (44).
The role of FDG-PET/CT in the management of GIST is unclear. Initial studies have shown that metabolic activity in GIST treated with imatinib declines dramatically on FDG-PET/CT and therefore FDG-PET/CT can be used for determining efficacy of drugs early in the treatment course (454647). However the routine use of FDG-PET/CT in clinical practice does not have additional advantages over CT scan (30). The National Comprehensive Cancer Network (NCCN) guidelines do not recommend FDG-PET/CT in the routine management of GIST (30). The European Society for Medical Oncology (ESMO) guidelines recommend FDG-PET/CT when targeted therapy is under investigation (48). FDG-PET/CT can be used to evaluate ambiguous findings encountered on CT or magnetic resonance imaging (MRI) but has no role in surveillance. MRI can be used as a problem solving tool for clarifying unusual responses on CT. Increase in tumor density in some GIST metastasis due to hemorrhage (especially with sunitinib) can mimic progression. MRI due to better soft tissue resolution can help in such scenarios (4).
The role of other advanced imaging techniques in the management of GIST is under research. Dual energy CT (DECT) scan allows visualization and quantification of iodine-related attenuation (IRA) and has the potential for accurate response assessment in GIST (49). In a study of 17 patients with advanced GIST treated with TKIs, RECIST, Choi criteria, and DECT criteria were compared. Patients were classified per DECT criteria as non-responders in this study if there was > 20% increase in size and IRA, > 50% increase in size or IRA (49). Responders by both Choi criteria and DECT criteria were associated with long PFS. Both DECT criteria and RECIST predicted PFS and overall survival (OS) but only DECT criteria were able to differentiate responders and non-responders according to PFS and OS (49). Volume CT perfusion (CTP) imaging is a novel imaging technique which determines tumor perfusion (50). Highly vascularized tumors like GIST tend to have high perfusion parameters on CTP. A decrease in perfusion parameters following targeted therapy can confirm response in ambiguous cases of response (50). The role of CTP in the management of GIST is yet to be studied.
Though complete surgical resection is feasible in a substantial proportion of GISTs, relapses are common, especially with high-risk GISTs (2). Surveillance with imaging in these patients can help in timely detection of relapses. The two most common sites of recurrence are the liver and peritoneum. In patients with no evidence of disease, recurrences can be seen as new metastatic deposits in the liver and peritoneum where as patients with residual cystic metastases, recurrence can be seen as increase in size or density of cystic lesions or as new intratumoral nodules referred to as 'nodule within mass' pattern of progression (Fig. 5) (51).
The optimal strategy for imaging surveillance is uncertain and can be guided by risk stratification using anatomic site of origin, tumor size and mitotic count. There are no established guidelines for the frequency of surveillance imaging in GIST. In patients who have resectable localized GIST, the NCCN recommends performing CT arbitrarily at intervals of 3–6 months for 3–5 years and then annually in the adjuvant setting after resection of the primary with the aim of detecting local recurrences and distant metastases (30). However, in a recent study, Joensuu et al. (52) found that hazard adjusted follow-up CT recommendations can significantly decrease the number of scans by up to 30% compared to the NCCN recommendations without affecting the efficacy of recurrent tumor. The ESMO guidelines suggest tailoring the follow-up schedules according to the risk stratification (48). GISTs with very low-risk of recurrence are invariably cured by surgery and therefore do not need adjuvant imatinib or longitudinal imaging. While GISTs with low-risk (excluding tumors with high mitotic counts) can be followed with sparse imaging for 5 years at 6–12 month intervals, intermediate and high-risk GISTs need denser imaging for at least 13 years (48). The timing of scans for high-risk GISTs recommended by ESMO includes 3–6 month intervals for the first three years during adjuvant imatinib, then every three months for 2 years and every 6 months for another 3 years after cessation of imatinib. Annual imaging is recommended for another 5 years (48).
The last few decades have seen tremendous advances in the understanding of the molecular taxonomy and biologic behavior of GIST. There has been proportionate increase in the contribution of radiologists in the complex management strategies of these patients. Upfront knowledge of the mutational taxonomy and familiarity with the risk stratification models can help radiologists in appropriate interpretation of scans. The field of tumor response assessment in GIST continues to evolve with advances in imaging techniques. Awareness of NCCN and ESMO guidelines can help in planning surveillance strategies in patients with GIST.
Figures and Tables
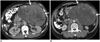 | Fig. 160-year-old man with gastric gastrointestinal stromal tumor with exon 11 mutation.
A. Axial contrast-enhanced CT image at time of diagnosis demonstrates large 13 cm gastric mass (arrows). Biopsy of mass revealed gastrointestinal stromal tumor with exon 11 mutation. Patient was treated with imatinib in neoadjuvant setting to downsize tumor. B. Follow-up CT after 3 months of imatinib therapy shows marked decrease in enhancing component in mass with no significant change in size (arrows). Patient underwent surgery with no evidence of recurrence at time of last follow-up 4 years later. CT = computed tomography
|
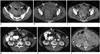 | Fig. 253-year-old woman with small bowel gastrointestinal stromal tumor with exon 9 mutation.
A. Axial contrast-enhanced CT image of lower abdomen reveals large cavitating mass in lower abdomen surrounded by small bowel loops (arrows). Patient underwent surgery which revealed small bowel mass. Histopathology revealed gastrointestinal stromal tumor with exon 9 mutation. B. Three months after surgery follow-up CT scan demonstrated recurrent pelvic mass (arrows). Patient was treated with high dose imatinib. C. CT scan after 6 months of treatment showed significant decrease in size of pelvic mass (arrows). Pelvic mass was excised and patient was restarted on high dose imatinib. D. Repeat CT scan after 4 months of treatment showed recurrence in form of multiple peritoneal masses (arrows). Patient was switched to sunitinib. E. CT scan performed 2 months after start of sunitinib therapy showed decrease in density of peritoneal deposits with mild increase in size (arrows). F. Follow-up CT scan three months later showed significant increase in peritoneal sarcomatosis. Patient died two months later. CT = computed tomography
|
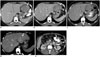 | Fig. 341-year-old man with gastric GIST with SDH mutation.
A. Axial contrast-enhanced CT image at time of diagnosis demonstrates large lobulated gastric mass (arrow). There is enlarged lymph node in gastrohepatic ligament (arrowhead). Patient was treated with neoadjuvant imatinib for one month. Follow-up CT scan showed no response to treatment and dose of imatinib was doubled. B. Repeat CT scan performed 3 months after therapy with high-dose imatinib showed no change in size of gastric mass and lymph node. Biopsy of mass at this time revealed SDH-deficient GIST. Patient was switched to sunitinib. C. CT scan after three months of treatment showed no response to treatment, instead new peritoneal nodule. Patient was taken up for surgery. D, E. Two years after surgery surveillance CT scan showed new liver metastasis (arrow, D) and peritoneal and bowel metastases (arrows, E). Patient was restarted on sunitinib. At time of last follow-up 7 years after initial diagnosis continues to have liver and peritoneal metastases which are stable in response to regorafenib therapy. CT = computed tomography, GIST = gastrointestinal stromal tumor, SDH = succinate dehydrogenase
|
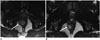 | Fig. 432-year-old man with anorectal gastrointestinal stromal tumor.
A. Axial T2-weighted MR image of pelvis reveals 3.3 cm mass (arrow) in anal canal. Biopsy of mass showed gastrointestinal stromal tumor with mitotic count of 1 per 50 high power fields. Tumor is low risk according to NIH consesus criteria, AFIP criteria and Joensuu criteria. Patient was treated with imatinib in neoadjuvant setting to downsize tumor. B. Follow-up MRI after 3 months of imatinib therapy shows decrease in size of mass (arrow). AFIP = Armed Forces Institute of Pathology, MRI = magnetic resonance imaging, NIH = National Institute of Health
|
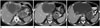 | Fig. 567-year-old man with gastric GIST metastatic to liver.
A. Axial contrast-enhanced CT image of abdomen at time of initial presentation reveals 8 cm partly necrotic gastric mass with irregular outline and enhancing solid internal component (arrow). There is large 14 cm necrotic liver mass consistent with metastasis (arrowhead). Presence of irregular outline and enhancing solid internal component on CT scan are predictive of increased risk of metastasis in GIST. Patient was treated with imatinib 400 mg. B. Follow-up CT after 12 months of imatinib therapy shows decrease in size and density of primary gastric mass (arrow) and also liver metastasis (arrowhead). C. Another CT scan performed 3 months later showed new enhancing nodule (balck arrowhead) in cystic liver metastasis (white arrowhead) consistent with recurrence. Primary gastric mass is again noted (arrow). Patient was switched to sunitinib. CT = computed tomography, GIST = gastrointestinal stromal tumor
|
References
1. Liegl B, Hornick JL, Lazar AJ. Contemporary pathology of gastrointestinal stromal tumors. Hematol Oncol Clin North Am. 2009; 23:49–68. vii–viii.
2. Call J, Walentas CD, Eickhoff JC, Scherzer N. Survival of gastrointestinal stromal tumor patients in the imatinib era: life raft group observational registry. BMC Cancer. 2012; 12:90.
3. Dematteo RP, Heinrich MC, El-Rifai WM, Demetri G. Clinical management of gastrointestinal stromal tumors: before and after STI-571. Hum Pathol. 2002; 33:466–477.
4. Tirumani SH, Jagannathan JP, Krajewski KM, Shinagare AB, Jacene H, Ramaiya NH. Imatinib and beyond in gastrointestinal stromal tumors: a radiologist’s perspective. AJR Am J Roentgenol. 2013; 201:801–810.
5. Hornick JL, Fletcher CD. The significance of KIT (CD117) in gastrointestinal stromal tumors. Int J Surg Pathol. 2004; 12:93–97.
6. Marrari A, Wagner AJ, Hornick JL. Predictors of response to targeted therapies for gastrointestinal stromal tumors. Arch Pathol Lab Med. 2012; 136:483–489.
7. Gronchi A. Risk stratification models and mutational analysis: keys to optimising adjuvant therapy in patients with gastrointestinal stromal tumour. Eur J Cancer. 2013; 49:884–892.
8. Wardelmann E, Büttner R, Merkelbach-Bruse S, Schildhaus HU. Mutation analysis of gastrointestinal stromal tumors: increasing significance for risk assessment and effective targeted therapy. Virchows Arch. 2007; 451:743–749.
9. Maleddu A, Pantaleo MA, Nannini M, Di Battista M, Saponara M, Lolli C, et al. Mechanisms of secondary resistance to tyrosine kinase inhibitors in gastrointestinal stromal tumours (review). Oncol Rep. 2009; 21:1359–1366.
10. O’Regan KN, Shinagare AB, Saboo SS, Ramaiya NH, Jagannathan JP, Tirumani SH. Gastrointestinal stromal tumors (GIST): lesser known facts. Clin Imaging. 2013; 37:821–829.
11. Tirumani SH, Tirumani H, Jagannathan JP, Shinagare AB, Hornick JL, George S, et al. MDCT features of succinate dehydrogenase (SDH)-deficient gastrointestinal stromal tumours. Br J Radiol. 2014; 87:20140476.
12. Antonescu CR, Sommer G, Sarran L, Tschernyavsky SJ, Riedel E, Woodruff JM, et al. Association of KIT exon 9 mutations with nongastric primary site and aggressive behavior: KIT mutation analysis and clinical correlates of 120 gastrointestinal stromal tumors. Clin Cancer Res. 2003; 9:3329–3337.
13. Miettinen M, Wang ZF, Sarlomo-Rikala M, Osuch C, Rutkowski P, Lasota J. Succinate dehydrogenase-deficient GISTs: a clinicopathologic, immunohistochemical, and molecular genetic study of 66 gastric GISTs with predilection to young age. Am J Surg Pathol. 2011; 35:1712–1721.
14. Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: a meta-analysis of 1,640 patients. J Clin Oncol. 2010; 28:1247–1253.
15. Heinrich MC, Corless CL, Demetri GD, Blanke CD, von Mehren M, Joensuu H, et al. Kinase mutations and imatinib response in patients with metastatic gastrointestinal stromal tumor. J Clin Oncol. 2003; 21:4342–4349.
16. Heinrich MC, Owzar K, Corless CL, Hollis D, Borden EC, Fletcher CD, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008; 26:5360–5367.
17. Tirumani SH, Jagannathan JP, Hornick JL, Ramaiya NH. Resistance to treatment in gastrointestinal stromal tumours: what radiologists should know. Clin Radiol. 2013; 68:e429–e437.
18. Heinrich MC, Corless CL, Blanke CD, Demetri GD, Joensuu H, Roberts PJ, et al. Molecular correlates of imatinib resistance in gastrointestinal stromal tumors. J Clin Oncol. 2006; 24:4764–4774.
19. Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002; 33:459–465.
20. Miettinen M, Lasota J. Gastrointestinal stromal tumors: pathology and prognosis at different sites. Semin Diagn Pathol. 2006; 23:70–83.
21. Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol. 2008; 39:1411–1419.
22. Rutkowski P, Bylina E, Wozniak A, Nowecki ZI, Osuch C, Matlok M, et al. Validation of the Joensuu risk criteria for primary resectable gastrointestinal stromal tumour - the impact of tumour rupture on patient outcomes. Eur J Surg Oncol. 2011; 37:890–896.
23. Goh BK, Chow PK, Yap WM, Kesavan SM, Song IC, Paul PG, et al. Which is the optimal risk stratification system for surgically treated localized primary GIST? Comparison of three contemporary prognostic criteria in 171 tumors and a proposal for a modified Armed Forces Institute of Pathology risk criteria. Ann Surg Oncol. 2008; 15:2153–2163.
24. Huang HY, Li CF, Huang WW, Hu TH, Lin CN, Uen YH, et al. A modification of NIH consensus criteria to better distinguish the highly lethal subset of primary localized gastrointestinal stromal tumors: a subdivision of the original high-risk group on the basis of outcome. Surgery. 2007; 141:748–756.
25. Gold JS, Gönen M, Gutiérrez A, Broto JM, García-del-Muro X, Smyrk TC, et al. Development and validation of a prognostic nomogram for recurrence-free survival after complete surgical resection of localised primary gastrointestinal stromal tumour: a retrospective analysis. Lancet Oncol. 2009; 10:1045–1052.
26. Chok AY, Goh BK, Koh YX, Lye WK, Allen JC Jr, Quek R, et al. Validation of the MSKCC gastrointestinal stromal tumor nomogram and comparison with other prognostication systems: single-institution experience with 289 patients. Ann Surg Oncol. 2015; 22:3597–3605.
27. Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: an analysis of pooled population-based cohorts. Lancet Oncol. 2012; 13:265–274.
28. O’Neill AC, Shinagare AB, Kurra V, Tirumani SH, Jagannathan JP, Baheti AD, et al. Assessment of metastatic risk of gastric GIST based on treatment-naïve CT features. Eur J Surg Oncol. 2016; 42:1222–1228.
29. Tirumani SH, Shinagare AB, Jagannathan JP, Krajewski KM, Ramaiya NH, Raut CP. Radiologic assessment of earliest, best, and plateau response of gastrointestinal stromal tumors to neoadjuvant imatinib prior to successful surgical resection. Eur J Surg Oncol. 2014; 40:420–428.
30. NCCN Clinical Practice Guidelines in Oncology. Soft Tissue Sarcoma Version 2. NCCN;2016. Accessed March 31, 2016. Web site. http://www.nccn.org/professionals/physician_gls/f_guidelines.asp#site.
31. Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schütte J, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: a randomized trial. JAMA. 2012; 307:1265–1272.
32. Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002; 347:472–480.
33. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006; 368:1329–1338.
34. Demetri GD, Reichardt P, Kang YK, Blay JY, Rutkowski P, Gelderblom H, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013; 381:295–302.
35. Kang YK, Ryu MH, Yoo C, Ryoo BY, Kim HJ, Lee JJ, et al. Resumption of imatinib to control metastatic or unresectable gastrointestinal stromal tumours after failure of imatinib and sunitinib (RIGHT): a randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2013; 14:1175–1182.
36. Choi H, Charnsangavej C, de Castro Faria S, Tamm EP, Benjamin RS, Johnson MM, et al. CT evaluation of the response of gastrointestinal stromal tumors after imatinib mesylate treatment: a quantitative analysis correlated with FDG PET findings. AJR Am J Roentgenol. 2004; 183:1619–1628.
37. Choi H, Charnsangavej C, Faria SC, Macapinlac HA, Burgess MA, Patel SR, et al. Correlation of computed tomography and positron emission tomography in patients with metastatic gastrointestinal stromal tumor treated at a single institution with imatinib mesylate: proposal of new computed tomography response criteria. J Clin Oncol. 2007; 25:1753–1759.
38. Rezai P, Pisaneschi MJ, Feng C, Yaghmai V. A radiologist’s guide to treatment response criteria in oncologic imaging: anatomic imaging biomarkers. AJR Am J Roentgenol. 2013; 201:237–245.
39. Tirumani SH, Shinagare AB, O’Neill AC, Nishino M, Rosenthal MH, Ramaiya NH. Accuracy and feasibility of estimated tumour volumetry in primary gastric gastrointestinal stromal tumours: validation using semiautomated technique in 127 patients. Eur Radiol. 2016; 26:286–295.
40. Schiavon G, Ruggiero A, Bekers DJ, Barry PA, Sleijfer S, Kloth J, et al. The effect of baseline morphology and its change during treatment on the accuracy of Response Evaluation Criteria in Solid Tumours in assessment of liver metastases. Eur J Cancer. 2014; 50:972–980.
41. Schiavon G, Ruggiero A, Schöffski P, van der Holt B, Bekers DJ, Eechoute K, et al. Tumor volume as an alternative response measurement for imatinib treated GIST patients. PLoS One. 2012; 7:e48372.
42. Schramm N, Englhart E, Schlemmer M, Hittinger M, Übleis C, Becker CR, et al. Tumor response and clinical outcome in metastatic gastrointestinal stromal tumors under sunitinib therapy: comparison of RECIST, Choi and volumetric criteria. Eur J Radiol. 2013; 82:951–958.
43. Shinagare AB, Barysauskas CM, Braschi-Amirfarzan M, O’Neill AC, Catalano PJ, George S, et al. Comparison of performance of various tumor response criteria in assessment of sunitinib activity in advanced gastrointestinal stromal tumors. Clin Imaging. 2016; 40:880–884.
44. Shinagare AB, Jagannathan JP, Kurra V, Urban T, Manola J, Choy E, et al. Comparison of performance of various tumour response criteria in assessment of regorafenib activity in advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib. Eur J Cancer. 2014; 50:981–986.
45. Van den Abbeele AD, Badawi RD, Tetrault RJ, Cliche JP, Manola J, Spangler T, et al. FDG-PET as a surrogate marker for response to Gleevec (TM) (imatinib mesylate) in patients with advanced gastrointestinal stromal tumors (GIST). J Nucl Med. 2003; 44:24–25.
46. Van den Abbeele AD for the GIST Collaborative PET Study Group. F18-FDG-PET provides early evidence of biological response to STI571 in patients with malignant gastrointestinal stromal tumors (GIST) [abstract]. Proc Am Soc Clin Oncol. 2001; 20:362a.
47. Van den Abbeele AD. The lessons of GIST--PET and PET/CT: a new paradigm for imaging. Oncologist. 2008; 13:Suppl 2. 8–13.
48. ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumours: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012; 23:Suppl 7. vii49–vii55.
49. Meyer M, Hohenberger P, Apfaltrer P, Henzler T, Dinter DJ, Schoenberg SO, et al. CT-based response assessment of advanced gastrointestinal stromal tumor: dual energy CT provides a more predictive imaging biomarker of clinical benefit than RECIST or Choi criteria. Eur J Radiol. 2013; 82:923–928.
50. Betz M, Kopp HG, Spira D, Claussen CD, Horger M. The benefit of using CT-perfusion imaging for reliable response monitoring in patients with gastrointestinal stromal tumor (GIST) undergoing treatment with novel targeted agents. Acta Radiol. 2013; 54:711–721.
51. Shankar S, vanSonnenberg E, Desai J, Dipiro PJ, Van Den Abbeele A, Demetri GD. Gastrointestinal stromal tumor: new nodule-within-a-mass pattern of recurrence after partial response to imatinib mesylate. Radiology. 2005; 235:892–898.
52. Joensuu H, Reichardt P, Eriksson M, Sundby Hall K, Vehtari A. Gastrointestinal stromal tumor: a method for optimizing the timing of CT scans in the follow-up of cancer patients. Radiology. 2014; 271:96–103.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download