INTRODUCTION
MV Anatomy
 | Fig. 1Normal CT images of MV.
A. Reconstructed short-axis view (en face view) showing each MV leaflet scallop (A1–A3 and P1–P3). In lateral portion of the mitral annulus, LAA and LCx are shown, and in anterior portion, aorta is seen. These landmarks are used for performing reconstruction in en face view of MV. B. Reconstructed three-chamber view (sagittal view) showing anterior leaflet (arrow) and posterior leaflet (dotted arrow) of MV. Fibrous continuity (arrowhead) is identified between aorta and anterior mitral leaflet. C. Reconstructed two-chamber view (coronal view) showing leaflets (arrow), chordae tendineae (dotted arrow), and papillary muscles (asterisks). Posterior leaflet of MV is divided into P1 (lateral), P2 (middle), and P3 (medial) scallop, respectively, opposing segments of anterior leaflet are named A1 (lateral), A2 (middle), and A3 (medial) scallops, respectively. AL = anterolateral commissure, Ao = aorta, CT = computed tomography, LA = left atrium, LAA = left atrial appendage, LCx = left circumflex artery, LV = left ventricle, MV = mitral valve, PM = posteromedial commissure
|
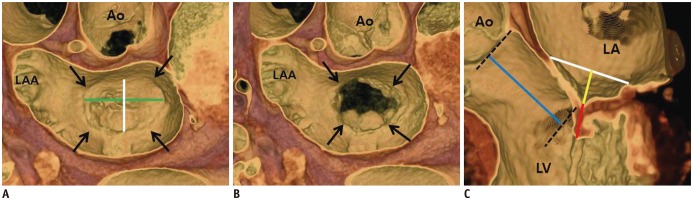 | Fig. 2MV coaptation geometry.
A, B. 3D volume-rendered CT images (en face view) showing mitral annulus (arrows). Intercommissural distance (green line) and septo-lateral distance (white line) can be measured in systolic (A) and diastolic phases (B). C. Geometric parameters such as septo-lateral distance (white line), tenting height (yellow line), coaptation length (red line), and lateral displacement of coaptation (blue line) can be observed and measured in three-chamber view of MV by using 3D volume-rendered CT image. 3D = three-dimensional
|
Mitral Annulus
MV Leaflets
Chordae Tendineae
Papillary Muscles
MV Coaptation Geometry
Carpentier Surgical Classification of MV Pathology
Table 1
Carpentier Surgical Classification of Pathology of Mitral Valve
MV Repair
Indications
Surgical Approaches
Artificial Neochordae
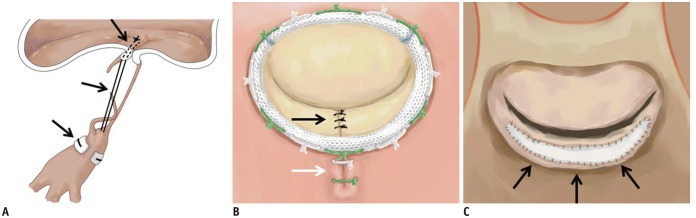 | Fig. 3Illustration of MV repair.
A. Artificial neochordae with polytetrafluoroethylene sutures (black arrows). B. Carpentier-Edwards Physio ring with quadrangular resection of posterior mitral leaflet (black arrow) and plication sutures of posterior mitral annulus (white arrow). C. Pericardial patch leaflet augmentation (black arrows).
|
Quadrangular and Triangular Resection of the Posterior Leaflet
Pericardial Patch Leaflet Augmentation
Commissuroplasty
Mitral Annuloplasty
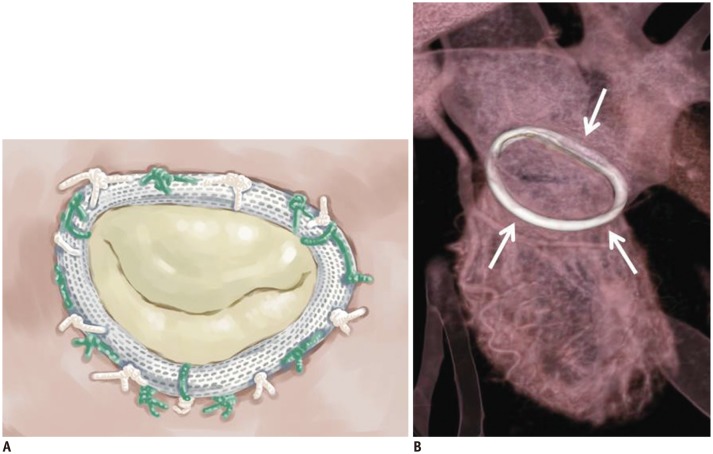 | Fig. 4Carpentier-Edwards Physio annuloplasty ring.Illustrative image (A) and CT image (B) showing high-density ring structure that surrounds annulus, i.e., Carpentier-Edwards Physio annuloplasty ring (white arrows).
|
 | Fig. 5Cosgrove-Edwards annuloplasty system.Illustrative image (A) and CT image (B) showing Cosgrove-Edwards annuloplasty system (black arrows) that partially surrounds mitral annulus.
|
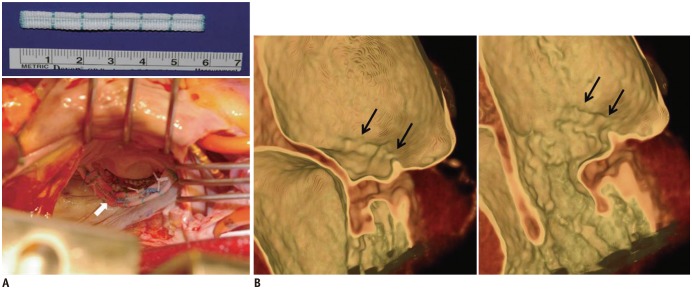 | Fig. 6Intraoperative photograph and post-operative CT images after posterior mitral annuloplasty with ML strip.
A. Intraoperative photograph showing posterior mitral annuloplasty strip (white arrow) placed on atrial wall 5.0 mm above posterior annulus, which decreased posterior annular circumference and spared anterior annulus and both commissures. B. Ridge-like lesion (black arrows) was identified on post-operative CT images during systolic and diastolic phases. ML strip = Mitra-Lift® strip
|
Pre-Operative and Post-Operative Imaging of the MV
CT Imaging Techniques
Imaging Reconstruction
MV CT Imaging
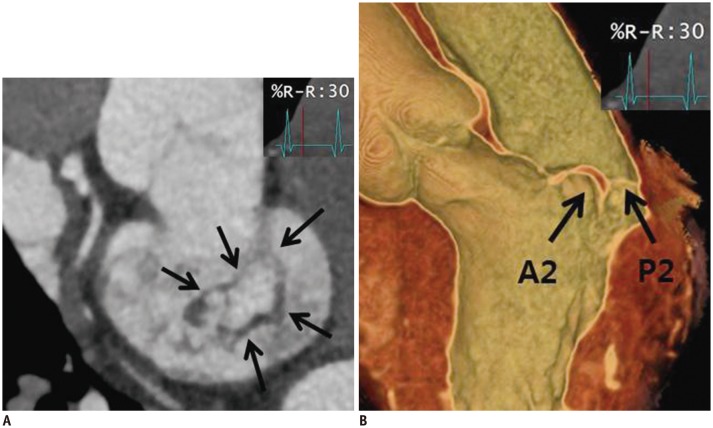 | Fig. 752-year-old man who presented with chest discomfort.Pre-operative CT images of MV. MV (arrows) was reconstructed by using multiplanar reformat display technique and 3D volume-rendered image with thin-slap reformation technique. En face view (A) and three-chamber view of 3D volume-rendered image (B) at level of MV during systolic phase showing prolapse at A2–A3 level of anterior mitral leaflet. Color Doppler echocardiography showed moderate degree of eccentric mitral regurgitation.
|
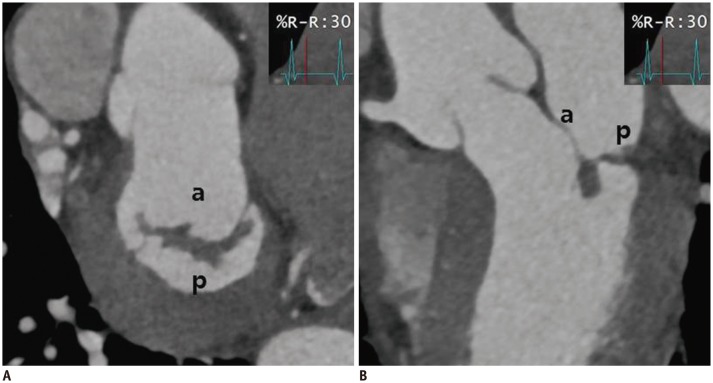 | Fig. 8Cardiac CT images after MV repair due to mitral regurgitation caused by MV prolapse.En face view (A) and three-chamber view (B) at level of mitral valve during systolic phase showing well functioning mitral valve without coaptation defect. a = anterior leaflet of MV, p = posterior leaflet of MV
|
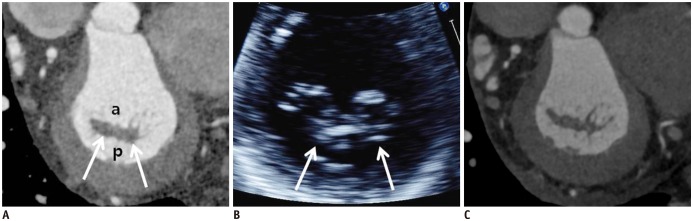 | Fig. 9Pre-operative and post-operative cardiac CT and echocardiographic images of 59-year-old man who presented with palpitation.
A. En face views at level of MV (white arrows) during systolic phase showing diffuse valvular thickening without definite mitral stenosis or regurgitation. Coaptation failure was not likely according to multiplanar reformatted images. B. Diffuse valvular thickening (white arrows) was also shown on echocardiography. Color Doppler echocardiography showed moderate degree of mitral regurgitation. C. Diffuse valvular thickening decreased after MV repair, but post-operative cardiac CT still showed small coaptation defect in MV.
|
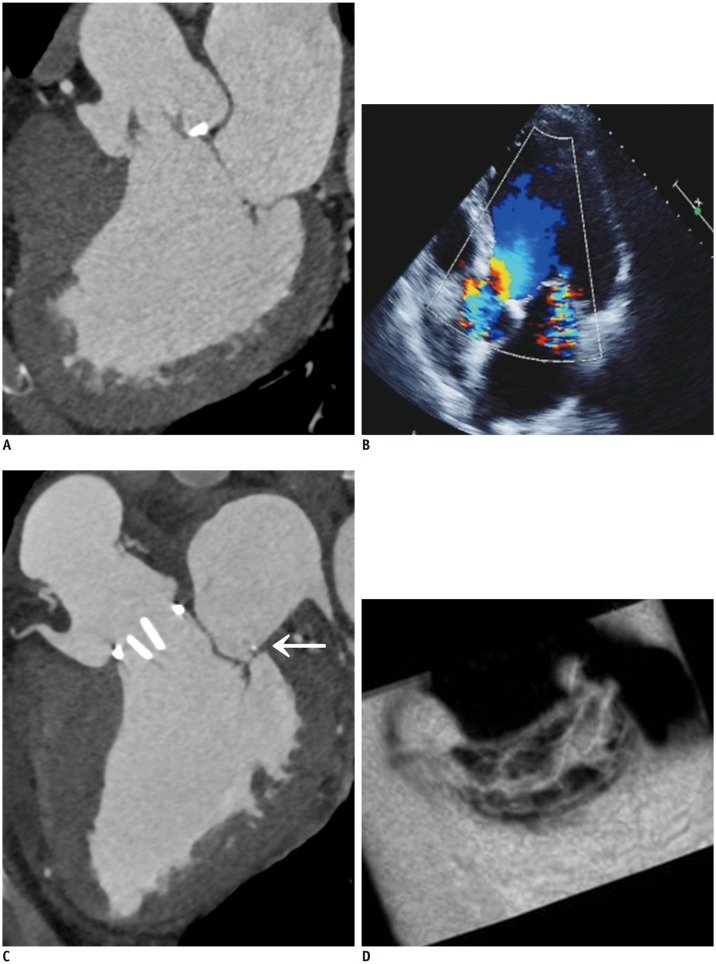 | Fig. 1063-year-old man who presented with exertional dyspnea.
A. Pre-operative cardiac CT of multiplanar reformatted images in three-chamber view of MV. B. Echocardiographic image is showing functional mitral regurgitation combined with bicuspid aortic valve with eccentric AR and aneurysmal dilatation of ascending aorta. C, D. Aortic valve replacement and ascending aortic aneurysm resection with graft interposition and Cosgrove-Edwards annuloplasty (C, arrow) were performed for functional mitral regurgitation. Post-operative cardiac CT of multiplanar reformatted images in three-chamber view (C) and en face view of MV (D). En face view of MV show well-functioning MV with good coaptation (D). AR = atrial regurgitation
|
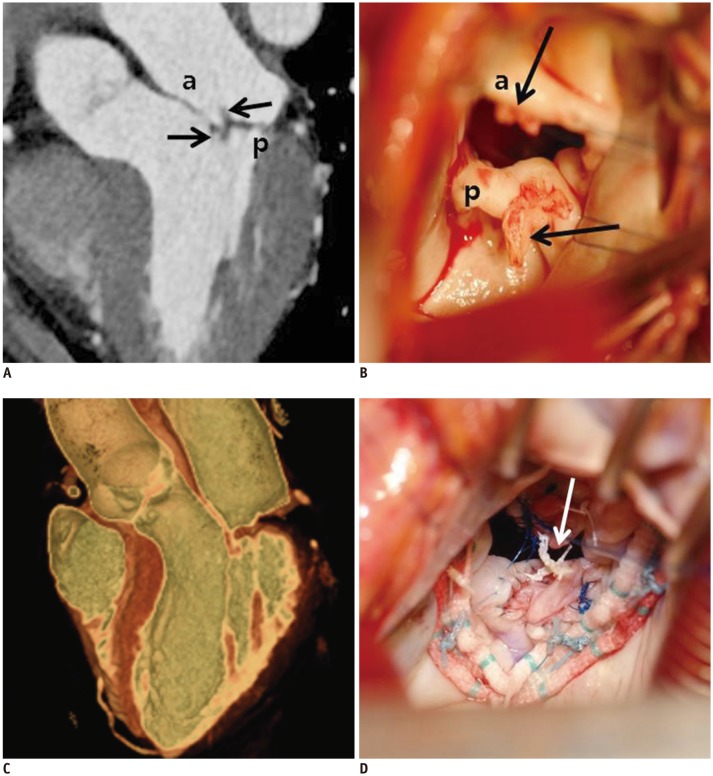 | Fig. 1157-year-old woman who presented with necrosis of left toe and finger.Pre-operative cardiac CT was obtained to evaluate MV and rule out infective endocarditis with vegetation.
A. Three-chamber view at level of MV was reconstructed. There were two small soft tissue density masses (black arrows) in anterior and posterior leaflets of MV with mild coaptation defect. B. Intraoperative photograph demonstrating two small masses (black arrows) in anterior and posterior leaflets of MV with endocarditis. Pre-operative echocardiography showed thickened MV with shaggy echogenic material on anterior and posterior mitral leaflets and mild mitral regurgitation. C. Cardiac CT after MV repair. MV was reconstructed by using 3D volume-rendered image with thin-slap reformation technique; well-functioning MV without coaptation defect was observed. D. Two small masses were removed with entire layers of leaflet. Leaflet defects were repaired with autologous pericardial patches and posterior mitral annuloplasty. Artificial neochordae (arrow) were replaced. Histological diagnosis of two small masses was confirmed as papillary fibroelastoma.
|
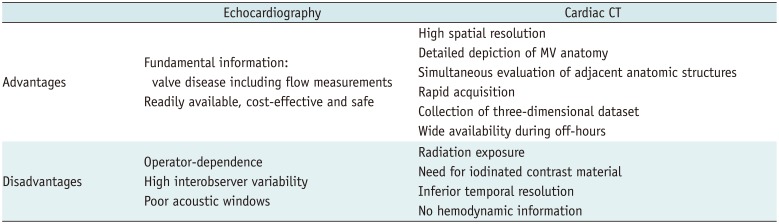
Table 2
Advantages and Disadvantages of MV Imaging between Echocardiography and Cardiac CT




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download