1. Berg AT, Shinnar S, Levy SR, Testa FM. Newly diagnosed epilepsy in children: presentation at diagnosis. Epilepsia. 1999; 40:445–452. PMID:
10219270.

2. Bien CG, Szinay M, Wagner J, Clusmann H, Becker AJ, Urbach H. Characteristics and surgical outcomes of patients with refractory magnetic resonance imaging-negative epilepsies. Arch Neurol. 2009; 66:1491–1499. PMID:
20008653.

3. Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001; 345:311–318. PMID:
11484687.

4. French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, et al. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993; 34:774–780. PMID:
8250525.

5. Gaillard WD, Chiron C, Cross JH, Harvey AS, Kuzniecky R, Hertz-Pannier L, et al. Guidelines for imaging infants and children with recent-onset epilepsy. Epilepsia. 2009; 50:2147–2153. PMID:
19389145.

6. Berg AT, Testa FM, Levy SR, Shinnar S. Neuroimaging in children with newly diagnosed epilepsy: a community-based study. Pediatrics. 2000; 106:527–532. PMID:
10969098.
7. See SJ, Jehi LE, Vadera S, Bulacio J, Najm I, Bingaman W. Surgical outcomes in patients with extratemporal epilepsy and subtle or normal magnetic resonance imaging findings. Neurosurgery. 2013; 73:68–76. discussion 76-77. PMID:
23615090.

8. Capraz IY, Kurt G, Akdemir Ö, Hirfanoglu T, Oner Y, Sengezer T, et al. Surgical outcome in patients with MRI-negative, PET-positive temporal lobe epilepsy. Seizure. 2015; 29:63–68. PMID:
26076845.

9. Téllez-Zenteno JF, Hernández Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010; 89:310–318. PMID:
20227852.

10. Winston GP, Micallef C, Kendell BE, Bartlett PA, Williams EJ, Burdett JL, et al. The value of repeat neuroimaging for epilepsy at a tertiary referral centre: 16 years of experience. Epilepsy Res. 2013; 105:349–355. PMID:
23538269.

11. Nguyen DK, Rochette E, Leroux JM, Beaudoin G, Cossette P, Lassonde M, et al. Value of 3.0 T MR imaging in refractory partial epilepsy and negative 1.5 T MRI. Seizure. 2010; 19:475–478. PMID:
20673641.
12. Knake S, Triantafyllou C, Wald LL, Wiggins G, Kirk GP, Larsson PG, et al. 3T phased array MRI improves the presurgical evaluation in focal epilepsies: a prospective study. Neurology. 2005; 65:1026–1031. PMID:
16217054.

13. Yoshida F, Morioka T, Hashiguchi K, Miyagi Y, Nagata S, Yamaguchi Y, et al. Appearance of focal cortical dysplasia on serial MRI after maturation of myelination. Childs Nerv Syst. 2008; 24:269–273. PMID:
17680248.

14. Yagishita A, Arai N, Maehara T, Shimizu H, Tokumaru AM, Oda M. Focal cortical dysplasia: appearance on MR images. Radiology. 1997; 203:553–559. PMID:
9114120.

15. Takanashi J, Barkovich AJ. The changing MR imaging appearance of polymicrogyria: a consequence of myelination. AJNR Am J Neuroradiol. 2003; 24:788–793. PMID:
12748072.
16. Sankar R, Curran JG, Kevill JW, Rintahaka PJ, Shewmon DA, Vinters HV. Microscopic cortical dysplasia in infantile spasms: evolution of white matter abnormalities. AJNR Am J Neuroradiol. 1995; 16:1265–1272. PMID:
7677022.
17. Eltze CM, Chong WK, Bhate S, Harding B, Neville BG, Cross JH. Taylor-type focal cortical dysplasia in infants: some MRI lesions almost disappear with maturation of myelination. Epilepsia. 2005; 46:1988–1992. PMID:
16393166.

18. Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia. 2010; 51:676–685. PMID:
20196795.

19. Vattipally VR, Bronen RA. MR imaging of epilepsy: strategies for successful interpretation. Neuroimaging Clin N Am. 2004; 14:349–372. PMID:
15324853.

20. Barkovich AJ, Kuzniecky RI, Jackson GD, Guerrini R, Dobyns WB. Classification system for malformations of cortical development: update 2001. Neurology. 2001; 57:2168–2178. PMID:
11785496.

21. Jackson GD, Berkovic SF, Tress BM, Kalnins RM, Fabinyi GC, Bladin PF. Hippocampal sclerosis can be reliably detected by magnetic resonance imaging. Neurology. 1990; 40:1869–1875. PMID:
2247236.

22. Wald LL, Carvajal L, Moyher SE, Nelson SJ, Grant PE, Barkovich AJ, et al. Phased array detectors and an automated intensity-correction algorithm for high-resolution MR imaging of the human brain. Magn Reson Med. 1995; 34:433–439. PMID:
7500883.

23. McBride MC, Bronstein KS, Bennett B, Erba G, Pilcher W, Berg MJ. Failure of standard magnetic resonance imaging in patients with refractory temporal lobe epilepsy. Arch Neurol. 1998; 55:346–348. PMID:
9520008.

24. Von Oertzen J, Urbach H, Jungbluth S, Kurthen M, Reuber M, Fernández G, et al. Standard magnetic resonance imaging is inadequate for patients with refractory focal epilepsy. J Neurol Neurosurg Psychiatry. 2002; 73:643–647. PMID:
12438463.

25. Barkovich AJ. Concepts of myelin and myelination in neuroradiology. AJNR Am J Neuroradiol. 2000; 21:1099–1109. PMID:
10871022.
26. Davies KG, Hermann BP, Dohan FC Jr, Foley KT, Bush AJ, Wyler AR. Relationship of hippocampal sclerosis to duration and age of onset of epilepsy, and childhood febrile seizures in temporal lobectomy patients. Epilepsy Res. 1996; 24:119–126. PMID:
8796360.

27. Tasch E, Cendes F, Li LM, Dubeau F, Andermann F, Arnold DL. Neuroimaging evidence of progressive neuronal loss and dysfunction in temporal lobe epilepsy. Ann Neurol. 1999; 45:568–576. PMID:
10319878.

28. Kadom N, Tsuchida T, Gaillard WD. Hippocampal sclerosis in children younger than 2 years. Pediatr Radiol. 2011; 41:1239–1245. PMID:
21735179.
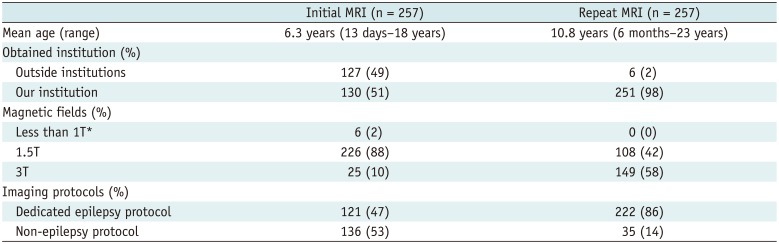


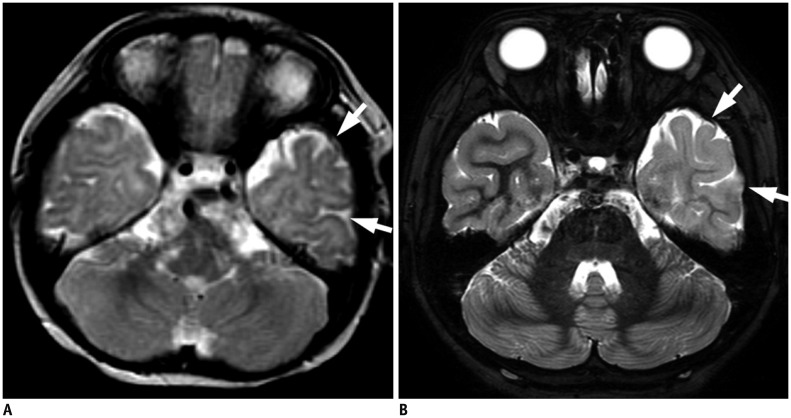
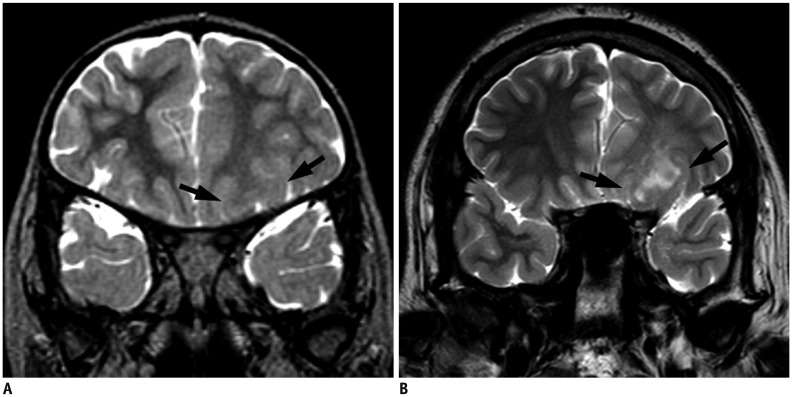
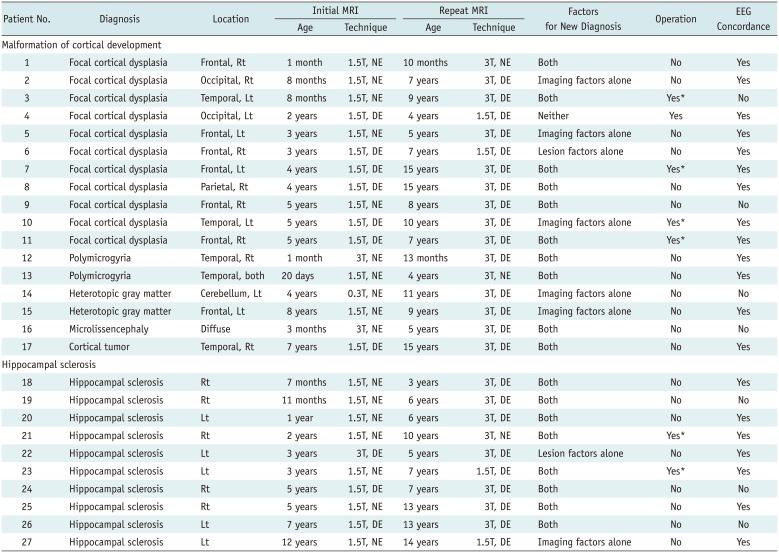




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download