INTRODUCTION
Normal Anatomy and Development
Congenital Lesions
Agenesis: Complete vs. Partial
Lipoma
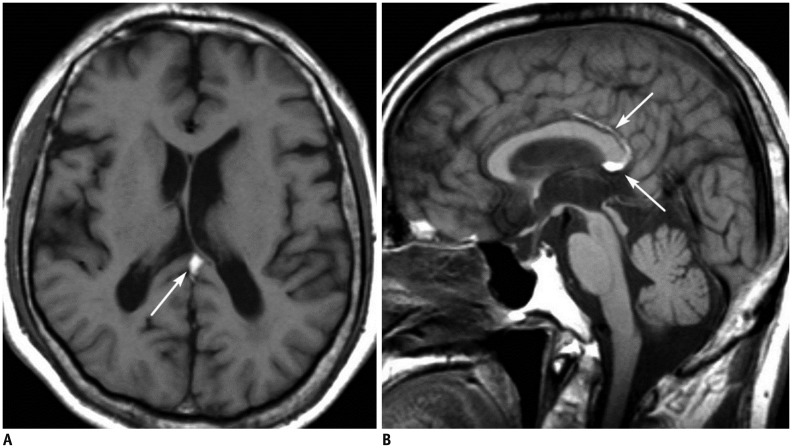
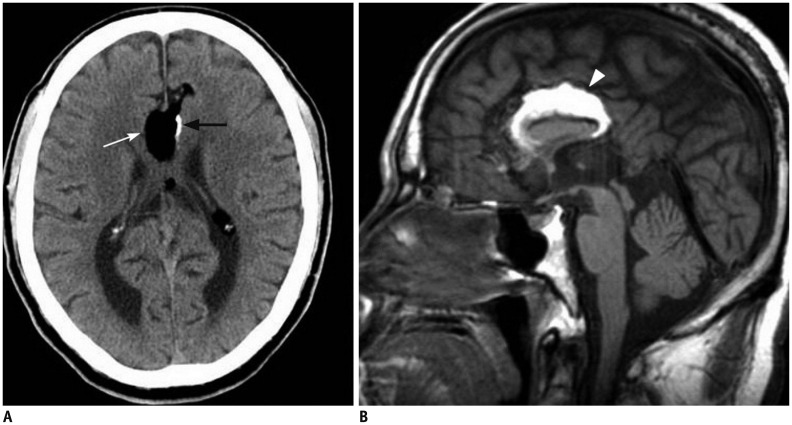 | Fig. 270-year-old male patient with tubulonodular type pericallosal lipoma and partial corpus callosal agenesis.
A. Axial CT image shows tubulonodular type lipoma in frontal interhemispheric fissure (white arrow). There is linear calcification at left side of lesion (black arrow). B. Sagittal T1-weighted MR image reveals partial agenesis of corpus callosum and hyperintense pericallosal lipoma (arrowhead).
|
Traumatic Lesions
Diffuse Axonal Injury
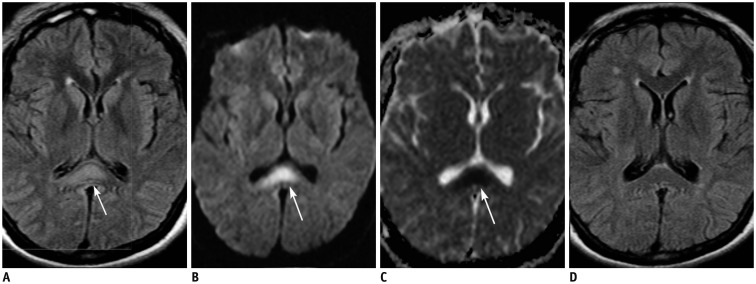 | Fig. 429-year-old female patient with diffuse axonal injury lesion.Patient was involved in motor vehicle accident 9 days ago.
A, B. Axial FLAIR (A) and DWI (B) images show hyperintense lesion in splenial portion of corpus callosum (arrows). Lesion is more conspicuously demonstrated on DWI than on FLAIR image. C. ADC map image reveals restricted water diffusion of lesion (arrow). D. On follow-up FLAIR image obtained 17 months later, splenial lesion has disappeared. ADC = apparent diffusion coeffcient, DWI = diffusion-weighted image, FLAIR = fluid-attenuated inversion recovery
|
Ischemic Lesions
Infarction
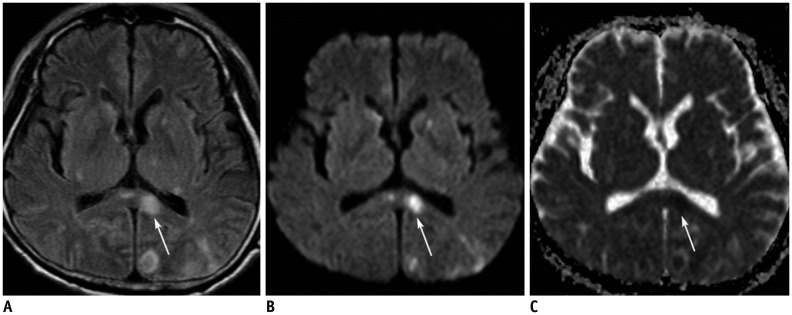 | Fig. 562-year-old male patient with acute splenial infarction.
A, B. Axial FLAIR image (A) and DWI (B) show multiple hyperintense lesions in bilateral basal ganglia, left thalamus, splenium of corpus callosum (arrows), and left occipital lobe. Splenial lesion (arrows) is more conspicuously demonstrated on DWI than on FLAIR image. C. ADC map image reveals restricted water diffusion of lesion (arrow). ADC = apparent diffusion coeffcient, DWI = diffusion-weighted image, FLAIR = fluid-attenuated inversion recovery
|
Hypoxic-Ischemic Encephalopathy
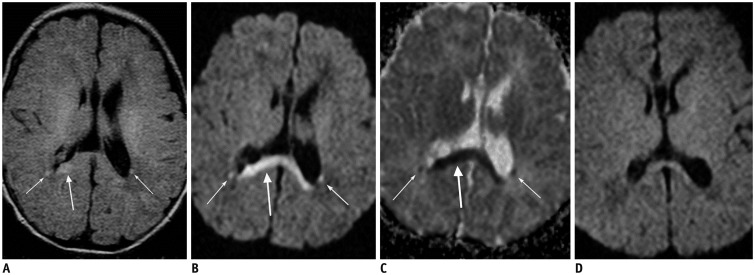 | Fig. 68-day-old female patient with hypoxic-ischemic encephalopathy.Patient had birth asphyxia.
A, B. Axial FLAIR image (A) and DWI (B) show hyperintense lesions in splenium of corpus callosum (thick arrows) and bilateral posterior deep periventricular white matter (thin arrows). C. ADC map image reveals restricted water diffusion of lesions (arrows). D. Follow-up axial DWI image obtained 1 month later shows decrease in signal intensity of lesions. ADC = apparent diffusion coeffcient, DWI = diffusion-weighted image, FLAIR = fluid-attenuated inversion recovery
|
Hypoglycemic Encephalopathy
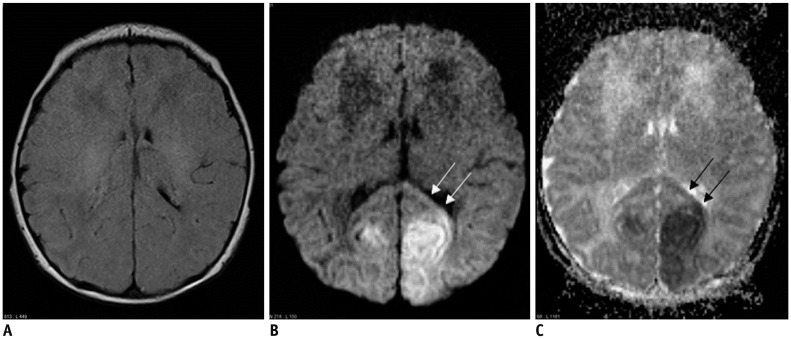 | Fig. 72-day-old female with hypoglycemic encephalopathy.Glucose level was 2 mg/dL at presentation.
A. On FLAIR axial image, there is no definite lesion. B. Axial DWI shows hyperintense lesions in both occipital lobes and splenium (arrows). C. ADC map image reveals restricted water diffusion of lesions (arrows). ADC = apparent diffusion coeffcient, DWI = diffusion-weighted image, FLAIR = fluid-attenuated inversion recovery
|
CO-Intoxication
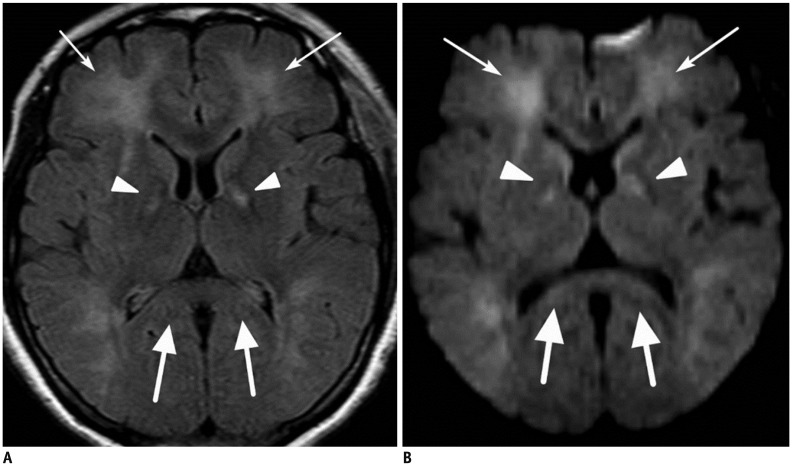 | Fig. 848-year-old female patient with CO-intoxication.Axial FLAIR image (A) and DWI (B) show multiple hyperintense lesions in bilateral globus pallidus (arrowheads) and cerebral white matter (thin arrows) including splenium of corpus callosum (thick arrows). DWI = diffusion-weighted image, FLAIR = fluid-attenuated inversion recovery
|
Tumors
Lymphoma
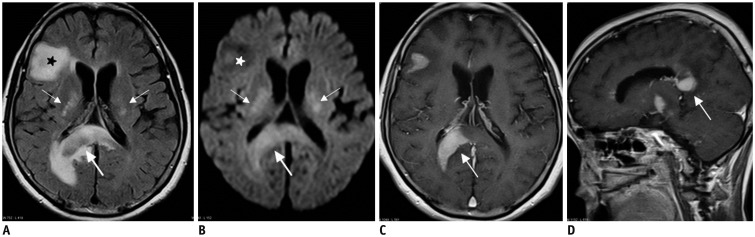 | Fig. 961-year-old female patient with lymphomas.
A, B. Axial FLAIR image (A) and DWI (B) show multiple hyperintense lesions in bilateral basal ganglia (thin arrows), splenium (thick arrows), and right frontal lobe (stars). C, D. On enhanced T1-weighted axial (C) and sagittal (D) images, splenial lesion (thick arrows) is homogeneously enhanced. DWI = diffusion-weighted image, FLAIR = fluid-attenuated inversion recovery
|
Glioma
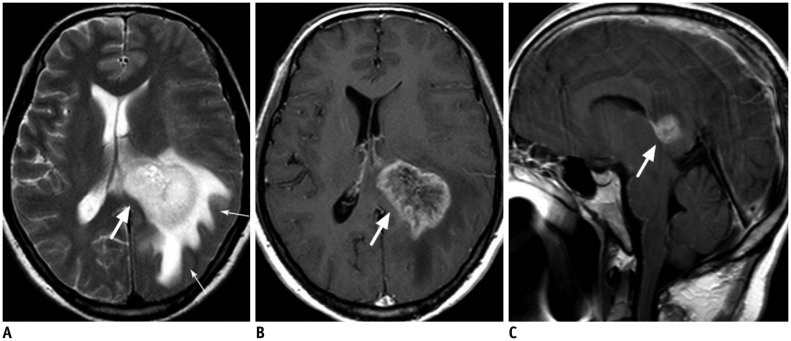 | Fig. 1052-year-old male patient with glioblastoma.
A. Axial T2-weighted image shows large hyperintense mass in left deep periventricular white matter and adjacent splenium of corpus callosum (thick arrow). There is large amount of surrounding brain edema (thin arrows). B, C. On enhanced T1-weighted axial (B) and sagittal (C) images, mass is strongly enhanced (thick arrows).
|
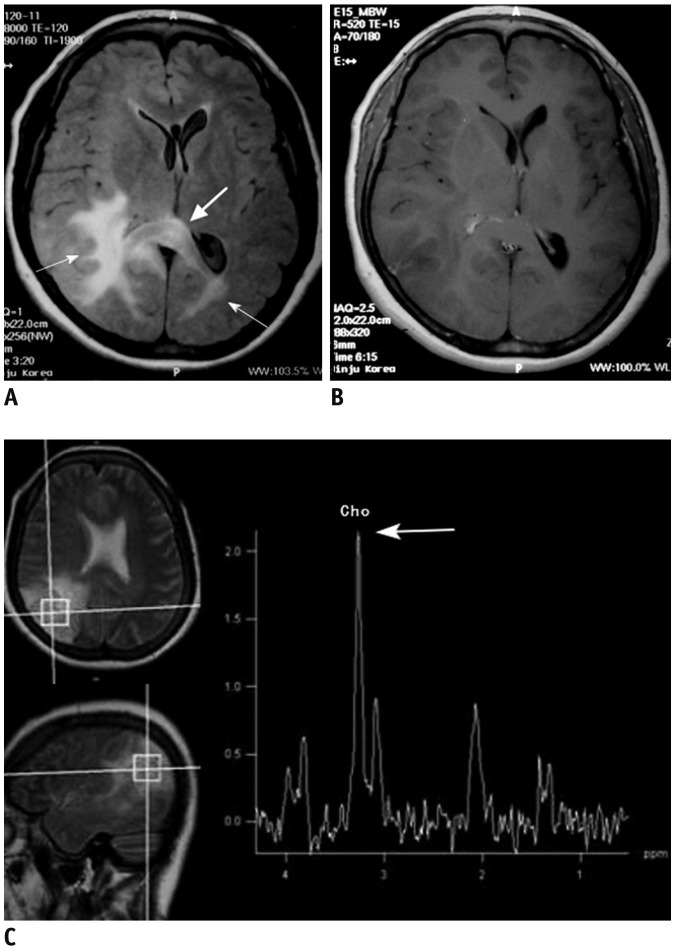 | Fig. 1146-year-old female patient with gliomatosis cerebri.
A. Axial FLAIR image shows ill-defined hyperintense lesions involving bilateral posterior cerebral white matter (thin arrows) and splenium of corpus callosum (thick arrow). B. Enhanced T1-weighted axial image shows no definite contrast enhancement of lesion. C. Single voxel 1H MR spectroscopy reveals increased choline peak (thick arrow). FLAIR = fluid-attenuated inversion recovery
|
Germinoma
Degenerative and Demyelinating Disease
Wallerian Degeneration
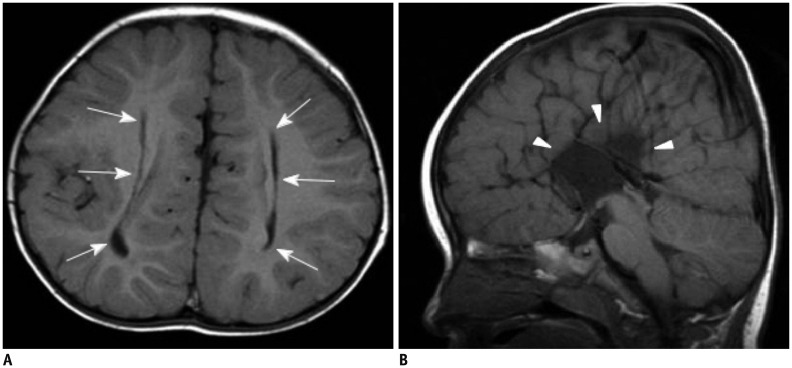
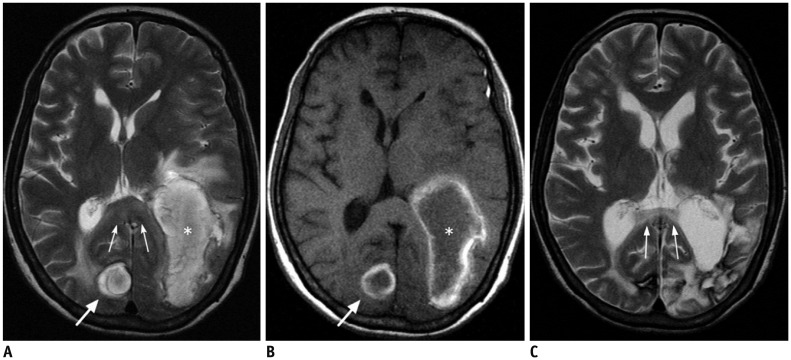 | Fig. 1349-year-old female patient with Wallerian degeneration due to intracerebral hematomas.
A, B. Axial T2 (A) and T1-weighted (B) images show two hyperintense hematomas in right occipital lobe (thick arrows) and left temporooccipital lobes (asterisks). There is moderate amount of surrounding brain edema. Splenium also shows mild swelling and increased signal intensity (thin arrows). C. Follow-up T2-weighted image obtained 20 months later reveals atrophic change and increased signal intensity of splenium (thin arrows).
|
Multiple Sclerosis
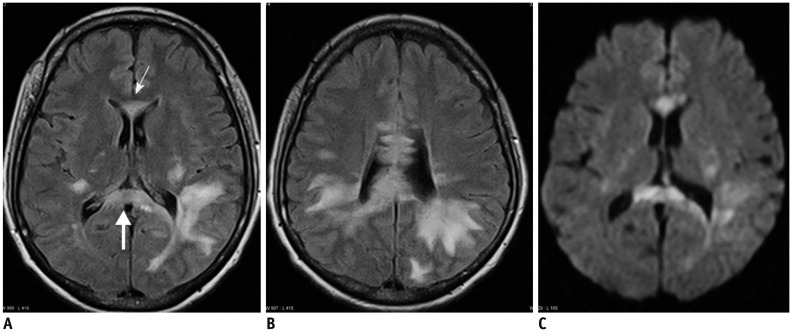 | Fig. 1439-year-old female patient with multiple sclerosis.
A, B. Axial FLAIR images show multiple hyperintense lesions in both periventricular white matter, genu (thin arrow), and splenium (thick arrow) of corpus callosum. C. On DWI, most of lesions are demonstrated as hyperintensities. DWI = diffusion-weighted image, FLAIR = fluid-attenuated inversion recovery
|
Acute Disseminated Encephalomyelitis
Posterior Reversible Encephalopathy Syndrome
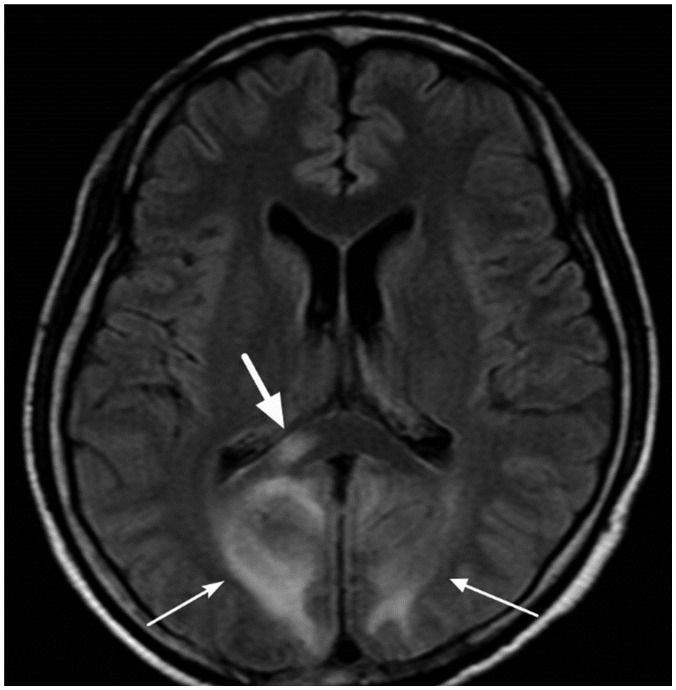 | Fig. 1529-year-old female patient with posterior reversible encephalopathy syndrome due to eclampsia.Axial FLAIR image shows multiple hyperintense lesions in cortices and white matter of both occipital lobes (thin arrows). There is also focal hyperintense lesion in right portion of splenium (thick arrow). FLAIR = fluid-attenuated inversion recovery
|
Toxic Encephalopathy
Marchiafava-Bignami Disease
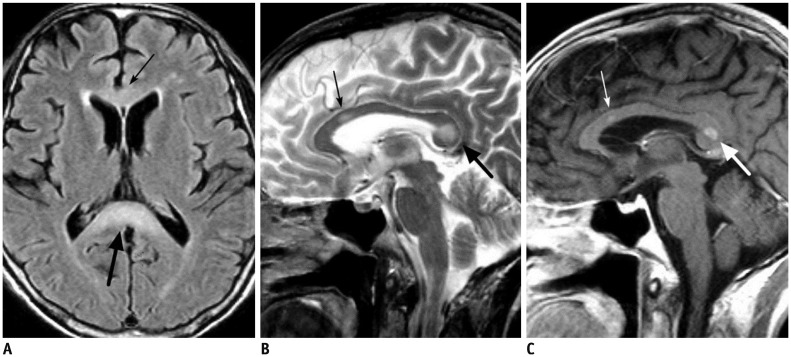 | Fig. 1659-year-old male patient with chronic alcoholism.
A, B. FLAIR (A) and T2-weighted (B) images show hyperintense lesions in body (thin arrows) and splenial portion (thick arrows) of corpus callosum. C. There is focal contrast enhancement of lesions (arrows) on post-contrast T1-weighted image. FLAIR = fluid-attenuated inversion recovery
|
Metronidazole-Induced Encephalopathy
Miscellaneous Lesions
Transient Lesion of the Splenium
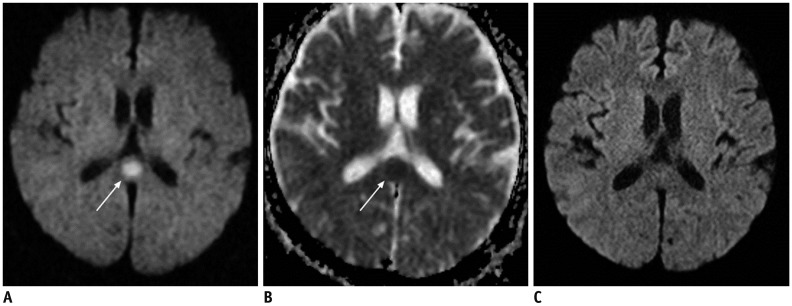 | Fig. 1762-year-old male patient with epilepsy.
A. Axial DWI shows focal hyperintense lesion in splenium of corpus callosum (arrow). B. ADC map image reveals restricted water diffusion of lesion (arrow). C. On follow-up DWI obtained 14 days later, splenial lesion has disappeared. ADC = apparent diffusion coeffcient, DWI = diffusion-weighted image
|
White Matter Injury in Neonatal Viral Infection
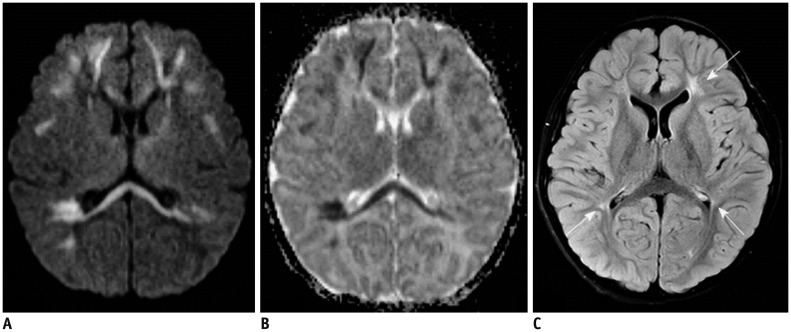 | Fig. 185-day-old male with rotavirus-related white matter injury.
A, B. Initial axial DWI (A) and ADC map (B) demonstrate extensive areas of restricted diffusion in periventricular white matter, deep white matter, corpus callosum, internal capsule, and posterior thalami. C. Follow-up FLAIR image obtained five years later shows residual ischemic lesions due to previous white matter injury in both periventricular white matters (arrows). ADC = apparent diffusion coeffcient, DWI = diffusion-weighted image, FLAIR = fluid-attenuated inversion recovery
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download