Abstract
Diffuse large B cell lymphoma (DLBCL) is the most common histological subtype of Non-Hodgkin's lymphoma. As treatments continues to evolve, so do imaging strategies, and positron emission tomography (PET) has emerged as the most important imaging tool to guide oncologists in the diagnosis, staging, response assessment, relapse/recurrence detection,and therapeutic decision making of DLBCL. Other imaging modalities including magnetic resonance imaging (MRI), computed tomography (CT), ultrasound, and conventional radiography are also used in the evaluation of lymphoma. MRI is useful for nervous system and musculoskeletal system involvement and is emerging as a radiation free alternative to PET/CT. This article provides a comprehensive review of both the functional and morphological imaging modalities, available in the management of DLBCL.
Diffuse large B cell lymphoma (DLBCL) is the most common histological subtype of Non-Hodgkin's lymphoma (NHL) accounting for 30% of NHL (1). DLBCL is an aggressive lymphoma that affects patients of all ages, with a wide range of clinical presentations. The median age at presentation is 64 years, with a slight male preponderance, and upto 50% of patients present with advanced stage disease.
Patients with DLBCL typically present with a rapidly enlarging lymph nodal mass, commonly in the neck or abdomen. 30% of patients present with fever, night sweats and weight loss (B symptoms). Extranodal extramedullary disease occurs in up to 40% of patients with DLBCL (2). The gastrointestinal tract is the most common site of extranodal involvement; however, DLBCL can involve virtually any organ, including the central nervous system (CNS), salivary glands, nasal cavity and paranasal sinuses, thyroid, breast, lung, liver, adrenal glands, kidneys and genital organs. Black race, male sex, age at diagnosis > 60 years, advanced stage, and B symptoms at diagnosis are adverse prognostic factors (3).
(F-18)2-fluoro-2-deoxy-D-glucose (FDG) positron emission tomography (PET)/computed tomography (CT) is the cornerstone in lymphoma management and is used at various stages of disease treatment including initial staging, treatment planning, and response assessment. Other imaging modalities such as ultrasound (US), CT, and magnetic resonance imaging (MRI) are often used as complementary tools for the assessment of morphology and to aid biopsies.
This reviewarticle summarizes clinically relevant aspects of the molecular classification and imaging modalities used in DLBCL, updated CT and PET treatment response criteria, and recenttreatment options, which should help the reader in day-to-day lymphoma imaging practice.
The histological subtypes of DLBCL are molecularly heterogeneous, and arise from several distinct oncogenic pathways. The molecular pathogenesis of DLBCL primarily involves rearrangements of BCL6, BCL2, and MYC that ultimately lead to the transformation and expansion of a malignant clone of germinal or postgerminal B cell origin.Molecular analysis using gene expression classifies DLBCL into 3 main subtypes-germinal center B-cell-like (GCB) DLBCL, activated B-cell-like (ABC) DLBCL, and primary mediastinal B-cell lymphoma (PMBL) (Table 1) (4). The GCB variant arises from germinal centre B cells and is most commonly associated with BCL2 translocation, resulting in BCL6 expression (56). MYC rearrangement (MYC+) is seen in approximately 10% of patients with DLBCL and often correlates with GCB subtype and has an inferior prognosis (7). The ABC variant arises from post germinal centre and occurs due to constitutive NFκB (8) and B cell receptor signaling pathway activation (9). The PMBL variant arises from post thymic B cells and is characterized by chromosome 9p24 amplification and NFκB pathway activation (10). PMBL is similar to the nodular sclerosis variant of Hodgkin lymphoma (11). It usually affects young adults, with a mean age of 37 years, and has a slight female preponderance (12). Other rarer subtypes of DLBCL are gray zone lymphoma and double hit lymphoma (DHL). Gray zone lymphoma has features of Classical Hodgkinlymphoma and PMBL. It usually presents as a mediastinal mass, asdoes PMBL, and demonstrates histological, immunohistochemical and genetic similarities to Classical Hodgkin lymphoma and PMBL (13). Gray zone lymphoma can be a therapeutic challenge,as it does not respond as expected to standard treatment regimens. DHL is a rare, highly aggressive subtype of NHL demonstrating MYC rearrangement in addition to BCL2 and/or BCL6 rearrangements (as detected by FISH or standard cytogenetics). DHL is seen in 2–11% of patients with DLBCL, and has features intermediate between DLBCL and Burkitt's lymphoma. Patients usually present with poor prognostic factors, such as elevated lactate dehydrogenase (LDH), bone marrow and CNS involvement. DHL is highly aggressive and even with newer chemoimmunotherapy regimens or stem cell transplantation is associated with poor clinical outcomes (14). An area of active research is in the development of new therapeutic tools based on molecular genetics of the diseasein patients who fail standard treatment. Molecular imaging will be complementary to clinical evaluation in guiding the success of these new drugs.
Cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) chemotherapy has been the standard of care for patients with DLBCL since the 1990s (15). The most recent breakthrough in the treatment of DLBCL was the addition of Rituximab, a chimeric monoclonal antibody against CD 20 expressed on mature B cells, which the FDA approved for use in 1997; CHOP plus rituximab (R-CHOP) has become standard of care in patients with DLBCL (1617). Involved site radiotherapy is recommended for patients who are not candidates for any chemotherapy. Patients with CNS disease are treated with systemic methotrexate. Intrathecal methotrexate is useful in patients with CNS disease with leptomeningeal involvement.
In recent years, genomic and molecular biomarkers have taken precedence over conventional histological classification to guide the selection of therapy and development of newer molecular targeted agents. Newer small molecules and pathway inhibitors are in various stages of investigation for the treatment of refractory DLBCL. Chimeric antigen receptor T cells targeting CD19-expressing B cells have shown promise in B-cell lymphoid malignancies (181920). Bispecific antibodies and their derivatives are a group of drugs that have shown promise in the treatment of hematologic malignancies (212223). Unlike ordinary monoclonal antibodies, bispecific antibodies form a link between T cells and tumor cells which allows the T cells to exert cytotoxic activity on tumor cells. Blinatumomab, a bispecific antibody approved by the FDA in December 2014 for the treatment of relapsed and/or refractory B-ALL, hasshown therapeutic efficacy in patients with relapsed/refractory DLBCL (24).
Another exciting area of active research is immunotherapy. The immune-checkpoint axis serves to maintain selftolerance and prevent autoimmunity. Malignant cells commonly overexpress inhibitory proteins which, in turn, suppress T-cell-effector functions, such as cytotoxic T-lymphocyte-associated-protein 4 (CTLA-4) and programmed cell-death protein 1 (PD-1), thus leading to immune escape of the tumour (25). Ipilimumab, an immune checkpoint inhibitor that blocks CTLA-4, was approved by the FDA in March 2011 for the treatment of metastatic melanoma (26). Since then, many other immunotherapeutic agents have shown promising results in treatment of hematologic malignancies and metastatic solid tumors. According to a recent phase 1 trial, nivolumab-mediated PD-1 blockade is found to be highly effective in patients with relapsed or refractory HL and was given FDA approval in May 2016 (27). Pidilizumab, a PD-1 blocking antibody, has shown promising results after autologous haematopoietic stem cell transplant in patients with DLBCL (28).
Approximately one-third of patients develop relapse or refractory disease in spite of overall improvements in the treatment strategy of DLBCL, and this remains a major cause of morbidity and mortality (29). Relapsed/refractory DLBCL is typically treated with high dose chemotherapy followed by autologous stem cell transplant (high dose chemotherapy [HD-CT] with autologous stem cell transplant [ASCT]) (30).
Imaging in oncology has shifted from purely morphological to a combination of morphological and functional imaging, which has led to improvement in overall management and patient care. With the advent of PET and fusion technology, creating PET/CT, molecular and morphological information can be accessed together. FDG PET/CT imaging has become the cornerstone for managing patients with lymphoma. FDG is a glucose analogue witha half life of 110 minutes that is taken up through glucose transporters on the surface of metabolically active cells. It has been extensively studied for its role in the staging, response assessment and detection of recurrence in patients with lymphoma.
Though FDG-PET/CT is the imaging modality of choice in DLBCL, other modalities, including plain radiography, US, CT, and MRI are also used at various stages of management. In patients with mediastinal DLBCL, chest radiography is typically the initial imaging study performed forsymptomatic work up, and mayprovide the first indication of involvement of the mediastinum and lung parenchyma. CT is particularly useful for anatomical delineation of pathological lymph nodes and extranodal disease, for guiding biopsies of deep seated lymph nodes, particularly in the mediastinum or retroperitoneum, and in formulating treatment plans. MRI is useful in imaging central and peripheral nervous system involvement. US can be used to assess the size, shape, border and echogenicity of superficial lymph nodes, such as those in the cervical, axillary, and inguinal regions (3132). However, US is of limited value in the evaluation of deep lymph nodes, such as thosein the retroperitoneum or mediastinum. US is also useful for the evaluation of organ involvement in the abdomen (e.g., gallbladder, kidney) and in guiding biopsy of focal lesions, if present, in the liver, spleen (33) or lymph nodes. US guided core biopsy in head and neck lymphoma has a high diagnostic yield, and can obviate the need for surgical excisional biopsies (34).
In 2014, the International Conference on Malignant Lymphomas (ICMLs) published guidelines for the initial evaluation, staging, treatment,and response assessment for various subtypes of lymphoma (38). The guidelines recommend the use of excisional biopsy, if possible, or core needle biopsy for the diagnosis of lymphoma (38). The initial clinical evaluation of suspected lymphoma should include age, sex, and detailed history of B-symptoms such as fever, weight loss, night sweats, lymphadenopathy and fatigue. Laboratory examination should include standard blood work including CBC with differential, a comprehensive metabolic panel and serum LDH. Size of palpable lymph nodes and organomegaly, if any, should be noted. The National Comprehensive Cancer Network-International Prognostic Index (NCCN-IPI) used in risk stratification of lymphoma patients incorporates age, LDH, sites of involvement, Ann Arbor stage, and Eastern Cooperative Oncology Group performance status (39). Newly diagnosed patients with DLBCL are classified into 4 different risk groups (low, low-intermediate, high-intermediate, and high), using this risk model (40).
The new Lugano classification, which is the most recent system proposed for staging and response assessment of both HL and NHL, was developed following meetings of the ICML in 2011 and 2013. This classification represents a consensus statement of clinical experts in lymphoma, andrecommends a five-point scale for response assessment with FDG-PET/CT, based on visual analysis, thus formally including PET/CT as the imaging study for staging of FDG-avid lymphomas, including DLBCL (3941). The inclusion of PET findings results in a change in staging in 10–30% of cases as compared to CT scanalone, and accurate staging ensures appropriate treatment of patients (42). Obtaining a baseline PET/CT scan is important in assessing FDG avidity of tumor prior to begining therapy, so as to increase the accuracy of subsequent response assessment on follow up imaging. The report template should include sites of lymph node involvement, size of the largest lymph node in each group (longest and shortest diameter) and locoregional extension, including encasement of vasculature and FDG uptake in Standarized Uptake Value (SUV). Organ involvement in lymphoma may show focal areas of increased FDG uptake or diffuse abnormal uptake. The initial staging PET/CT scan assesses metabolic activity, but utilizes a low dose, non-enhanced CT scan for attenuation correction, as contrast may result in overestimation of reference site activity in the liver and mediastinum (43). However, it may be complemented with a contrast enhanced CT (CECT) scan for better assessment of morphological abnormalities. In abdominal lymphoma, CECT may be helpful in differentiating lymph nodes from collapsed bowel loops and vessels; in head and neck lymphoma, it may be useful to differentiate physiologic uptake from enlarged cervical lymph nodes. A diagnostic CT scan is required where precise measurements of involved lymph nodes are needed (44).
Evaluation of bone marrow involvement is an essential step in the staging of lymphoma, and is associated with advanced disease stage in most cases. FDG-PET is a non-invasive, sensitive modality for detecting bone marrow involvement, seen as focal areas of FDG uptake, often with no morphological abnormality on corresponding CT images. Diffuse FDG uptake in bone marrow is more likely to be benign, secondary to reactive marrow activation, rather than pathological marrow involvement.
In one study, FDG-PET showed higher sensitivity, accuracy and negative predictive value (94%, 98% and 98% respectively) when compared to bone marrow biopsy (BMB) (24%, 80% and 81%, respectively) (45). However, in another study, FDG-PET performed poorly as compared to BMB and missed bone marrow involvement in 31.2% of cases (46). A plausible reason for FDG-PET being negative may be the presence of low volume disease. Presently, the Lugano classification indicates that a BMB is no longer indicated for Hodgkin lymphoma if a PET/CT is performed and positive for marrow involvement. A BMB is only indicated for DLBCL patients if the PET is negative and/or identifying a discordant histology is important for patient management (38). If both the BMB and FDG-PET show bone marrow involvement, prognosis is worse; no adverse effect on outcome was found when only FDG-PET was positive and BMB was negative (47).
The first universally accepted response criteria for NHL were published in 1999 by the National Cancer Institute Working Group and revised in 2007 by the International Working Group (48). The current Lugano classification recommends use of FDG-PET/CT for assessment of treatment response (Table 2, Fig. 1) (38). FDG-PET/CT is better than CT in confirming complete resolution of disease or identifying those patients who fail standard treatment. Interim-PET (I-PET)/CTs are performed early during treatment, following 2 to 4 courses of chemotherapy. End of treatment FDG-PET/CT scan should be performed after 6–8 weeks of therapy (48). According to the Lugano guidelines, response to treatment is classified as complete, partial, no responseor progressive disease. The Deauville criteria, a 5-point PET scoring system,along with SUV max is used to determine the response to therapy (Fig. 2) (4249).
There is an additional score ‘X’ for new areas of uptake that are unrelated to lymphomatous disease.
A score of 1–3 indicates metabolic response, whereas a score of 4 or 5 either indicates stable disease or progressive disease at the end of treatment evaluation. However, a score of 4 or 5 at interim evaluation may suggest a partial metabolic response, provided uptake has reduced from baseline. In addition to Deauville criteria, ΔSUV max was proposed by an international confirmatory study and is found to be better in evaluating the prognostic value of an I-PET/CT in DLBCL (50). ΔSUV max is the percentage difference between baseline and I-PET/CT SUV measurement performed after 2 cycles. A ΔSUV max cut-off of 66% was used to separate patients as I-PET-positive from I-PET-negative. This method was found to have the highest interobserver reproducibility, with almost perfect agreement between pairs of observers and a higher outcome prediction performance as compared to the Deauville scoring system. It is thus recommended by the authors as a semiquantitative tool, in addition to visual analysis used with Deauville criteria, in the assessment of interval treatment response.
In a large prospective International Atomic Enery Agency (IAEA)-funded multicenter study, the role of I-PET was investigated by Carr et al. (51). They looked at event-free survival (EFS) and overall survival (OS) over 2 years, reporting 79% and 86%, respectively, across all groups. However, when divided into I-PET-negative and I-PET-positive groups, the two-year EFS was 90% and 58%, respectively, and the two-year OS was 93% and 72%, respectively. Three main conclusions were drawn from this study: 1) response assessed by I-PET is comparable across various healthcare systems internationally, 2) a negative I-PET with good clinical status identifies a favorable subset of patients with EFS of 98%, and 3) a single I-PET scan cannot differentiate chemoresistant lymphoma from complete response and thus cannot be used to guide changes in management.
The PET guided therapy of Aggressive NHLtrial (52) and GA 101 In Newly Diagnosed Diffuse Large B Cell Lymphoma study (GAINED) are on going trials employing use of ΔSUV max in evaluating the role of I-PET/CT in changing management. Results will further assist in determining the utility of I-PET.
In addition to treatment response, I-PET/CT may detect drug toxicity, when present. Rituximab, a mainstay in treatment of DLBCL which has changed the overall scenario of DLBCL treatment favorably, is one of several causes of drug induced pneumonitis (Fig. 3). Complement activation is one of the speculated mechanisms in the pathogenesis of the side-effects of rituximab (53). It presents on FDG-PET/CT as diffuse FDG uptake within the lungs; ground glass opacities are the most common morphological abnormality on CT. Other changes, such as nodular interstitial thickening and consolidation, may also be present (54). Identification of these changes may alert the treating oncologist and timely treatment may prevent a potentially fatal outcome.
A recent meta-analysisof seven studies comprising a total of 737 R-CHOP-treated DLBCL patients who were in complete remission at end-of-treatment FDG-PET revealed a disease relapse rate of 7–20%. The progression free survival at 2 years, 3 years and 5 years was 83%, 85–86.4% and 75%, respectively (55). A Deauville score of 3 in many patients indicates a good prognosis at the time of an interim scan with standard treatment, however, a score of 3 at completion of therapy should be considered an inadequate treatment response (4142). OS or progression free survival are the usual end points in clinical trials, and FDG-PET/CT response criteria correlate well with progression free survival. Those patients with complete metabolic responses show negligible relapse rates. Thus, using FDG-PET/CT as a surrogate end point in trials for new drug development may shorten the time of clinical trials, hastening the availability of new drugs which may benefit patients (425556).
Metabolic response on FDG-PET/CT is also considered an important prognostic parameter in patients receiving second line therapy. In a prospective study of 24 patients with NHL relapse, predictive value of FDG-PET was found to be better than conventional re-staging and the IPI (57). In another study of 129 patients with relapsed or refractory DLBCL, after salvage chemotherapy and prior to HD-CT with ASCT, patients with Deauville scores of 1–3 had better PFS and OS than those with Deauville scores of 4 (77% and 86% vs. 49% and 54%, respectively). In these patients, complete metabolic response on FDG-PET appeared to be a favorable prognostic factor; no other factors had an impact on survival (58). A meta-analysis of 12 studies with 630 patients (which included 313 DLBCL patients) showed that patients with relapse and post salvage chemotherapy and no response on PET scan had a 4–5 times higher risk for treatment failure than patients that had a metabolic response on PET scan. In general, most studies show that FDG-PET findings have an impact on prognosis and survival in patients with relapsed disease. Patients not showing response on FDG-PET scan can be considered for investigational therapy (58).
Although PET/CT remains the corner stone for initial diagnosis, staging and response assessment in patients with DLBCL, MRI plays an important role in selected patient populations. MRI is particularly useful in patients with nervous system, bone marrow and musculoskeletal system involvement (Fig. 3). MRI is the imaging modality of choice in the work up of pediatric and pregnant patients with DLBCL, due to risk of exposure to ionizing radiation in diagnostic modalities like CT and PET/CT.
Central nervous system involvement in DLBCL can be primary or secondary. Primary CNS lymphomas (PCNSL) are B-cell type NHL, confined to the cranio-spinal axis, and without systemic involvement. DLBCL accounts for more than 90% of PCNSL (5960). CNS involvement in DLBCL typically involves the brain parenchyma, with less frequent involvement of the spinal cord, leptomeninges, pituitary gland, cranial nerves and the globe (61). The term “neurolymphomatosis” is used for involvement of the peripheral nervous system by lymphoma. In a retrospective analysis of 50 patients by the International Primary CNS Lymphoma Collaborative Group, the neural structures which were affected by lymphoma included peripheral nerves in 60%, spinal nerve roots in 48%, cranial nerves in 46%, plexus in 40%, and multi-site involvement in 58% (62). On MRI, lymphomatous lesions are usually hypo- or isointense to grey matter on unenhanced T1-weighted sequences, and often hypointense to grey matter on T2-weighted sequences, due to high nuclear/cytoplasm ratio and hypercellularity. Most lesions show moderate to marked enhancement on contrast-enhanced T1-weighted images, with moderate surrounding vasogenic edema (Fig. 4). MRI is useful in patients with predominantly parenchymal involvement, as sensitivity of cerebrospinal fluid analysis is low in the absence of leptomeningeal disease. Secondary CNS involvment can occur in upto 40% of patients with high-grade NHL, particularly in settings of relapsed disease (63). Secondary CNS involvement in DLBCL occurs via hematogenous dissemination, meningeal involvement and direct extension. Leptomeningeal disease is more common in secondary CNS lymphoma than in the primary form. MRI findings in leptomeningeal involvement include leptomeningeal or dural enhancement, cranial nerve enlargement and enhancement, and hydrocephalus.
Although MRI is the diagnostic imaging modality of choice in PCNSL, FDG-PET will detect foci of involvement outside the brain in a significant number of patients. In a retrospective study of 166 PCNSL patients, 49 patients had body FDG-PET. NHL was found in 11% of all patients, in 7% at time of diagnosis, and in 27% associated with CNS relapse (64). Although thebrain is highly FDG-avid due to itsdependence on glucose for energy, DLBCL in the brain presentsas highly metabolic lesions that are more avid than the surrounding normal brain parenchyma. Smaller lesions may be missed, and thus MRI is always indicated in cases of brain involvement of lymphoma. PET-MRI or fusion imaging is helpful when only subtle foci of increased metabolic activity are noted on FDG-PET scans.
Diffusion weighted imaging (DWI) is a functional MRI technique which relies on differences in the mobility of water in different tissues. The corresponding apparent diffusion coefficient quantifies the mobility of water protons in tissues. Neoplasms, including lymphomas, exhibit more restricted water diffusion than normal tissues, which is reflected as high signal intensity on DWI. Whole-body MRI (WB-MRI) is a novel imaging technique which permits whole body imaging with high soft tissue resolution in a reasonable time frame using fast magnetic resonance pulse sequences. Although this initially involved acquisition of coronal short tau inversion recovery images of the whole body, WB-MRI has evolved to include multiple sequences, including T1-weighted and DWI. WB-MRI is usually performed on a 1.5 Tesla or higher field strength. 1.5T images have the benefit of lower specific absorption rate and lesser magnetic field homogeneities, while 3T images have theadvantage of higher SNR and better spatial and temporal resolution. The acquisition time of a WB-MRI is similar or less than a PET/CT examination and is generally well tolerated by patients.
In patients with FDG-avid lymphoma, including DLBCL, DW-MRI has a sensitivity of 97%, for staging of the disease and is only slightly inferior to FDG-PET/CT (65). According to a prospective study of 36 children with newly diagnosed lymphoma, WB-DWI-MRI demonstrated sensitivity and specificity of 93% and 98% for nodal staging and 89% and 100% for extranodal staging, respectively (66). The recent introduction of WB combined PET and MRI (PET/MRI) offers the dual advantage of high soft tissue resolution and functional information of MRI, as well as metabolic information of PET. In recent prospective comparative studies of FDG-PET/MRI and WB-DW-MRI, recearchers have found a similar performance and high concordance among both modalities in the clinical work-up of lymphomas (6768). PET/MRI can accurately assess nodal and extranodal disease in patients with lymphoma and has the potential to become a viable alternative for lymphoma staging and follow-up.
Diffuse large B cell lymphoma is the most common sub-type of human immunodeficiency virus (HIV)-associated lymphoma, seen in approximately 40% of patients; other common subtypes include Burkitt (21%) and Hodgkin (18%) lymphoma (Fig. 5) (69). The pathogenesis of DLBCL in the setting of HIV infection has been linked to low CD4 counts, high HIV viral loads and a high prevalence of oncogenic viruses, particularly Epstein-Barr virus. Acquired immune deficiency syndrome (AIDS) related lymphomas have higher prevalence of extranodal disease, involvement of unusual locations and often present as advanced (stage III/IV) disease at initial diagnosis (69). HIV-associated DLBCL has some differentiating imaging features when compared to DLBCL in the general population. CNS lymphoma in HIV infected patients usually presents with multifocal lesions and has a more heterogeneous appearance due to central necrosis. CECT or MRI typically shows irregular and inhomogeneous contrast enhancement that is unusual in the HIV-seronegative population (707172). In the thorax, solitary or multiple pulmonary nodules associated with cavitation are common imaging features of DLBCL in HIV patients. Associated mediastinal lymphadenopathy may also be seen (73). In the abdomen, HIV-related NHL is characterized by exuberant lymph node enlargement, along with a higher incidence of extranodal disease, including hepatic, splenic, omental and gastrointestinal tract involvement (74).
In an immunocompromised state, it is not always possible to differentiate infective pathology from neoplastic lymphomatous involvement on morphological imaging. Although PET imaging may have added value, FDG uptake maybe seen in both neoplastic and infective pathologies, and thus limits its utility. Newer PET tracers such as fluorothymidine (FLT) mayexploit the ability of the tumor cell to undergo continuous proliferation, which could be helpful in differentiating infection from neoplastic involvement (7576). FLT is a fluorine-18 labeled analog of thymidine which undergoes phosphorylation by thymidine kinase-1 (77). Tumor cells have high expression of thymidine kinase-1 activity and thus show increased FLT uptake. In a study of 55 patients with lung nodules, FDG and FLT scans were performed, and the sensitivity and specificity of this dual tracer combination to differentiate benign from malignant pathology was 100% and 89.7%, respectively (78). However, there are some conflicting results of FLT uptake seen in inflammatory and granulomatous disease (7980), and other tracers like Carbon-11 labeled methionine have been proposed for the differentiation of infection from neoplasm (79). Thus, combination dual tracer imaging may be useful in difficult scenarios, especially in patients with immumosupression, and in challenging situations, image-guided biopsy maybe performed.
Richter's transformation (Richter's syndrome) is an uncommon clinicopathological condition seen in about 5–10% of patients with chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma, characterized by transformation into an aggressive lymphoma, usually DLBCL (Fig. 6) (81). Clinically, it is characterized by rapidly enlarging lymph nodes, hepatosplenomegaly, worsening “B” symptoms (fever, night sweats, and weight loss) and elevated serum LDH levels. PET/CT is useful in detecting lymph nodes having Richter's transformation in CLL patients with both high sensitivity and negative predictive values (82). In a retrospective study of 37 patients with CLL, using a diagnostic cutoff of SUV greater than 5, PET/CT had overall sensitivity, specificity, and positive and negative predictive values of 91%, 80%, and 53% and 97%, respectively (83). FDG-PET is also useful in assessing prognosis in cases of Richter's transformation. In a study of 332 patients with CLL, using PET/CT and histopathology, Falchi and coworkers found that patients with Richter's transformation had higher SUVmax compared to histologically indolent CLL (84). An SUV > 10 was associated with poor survival. In patients with suspected Righter's transformation, biopsy should aim to sample a lymph node with the highest metabolic activity on PET.
Intravascular large B-cell lymphoma (IVLBCL) is a rare subtype of extra nodal large B cell lymphoma, where there is proliferation of lymphoma cells into the vascular lumen without involvement of surrounding organ parenchyma (85). It typically occurs in elderly persons and can involve vasculature of any organ, commonly the brain and the skin (86). IVLBCL is classified into the classical, visceral form of the disease (also referred to as the “Western form” of IVLBCL), a cutaneous variant, and an Asian variant (predominant bone marrow, liver, lungs and adrenal gland involvement) (87). The prognosis of IVLBCL is extremely poor (88). Contrast-enhanced MRI is the single most useful imaging modality to diagnose intravacular CNS lymphoma. On MR images it appears as multiple small infarcts and can be confused with CNS vasculitis. Other findings include nonspecific white matter lesions in the periventricular regions and pons, masslike lesions, and meningeal enhancement (89).
Surveillance in any disease is to detect disease relapse before it manifests clinically or before the patient becomes symptomatic. In Stage I and II DLBCL, the NCCN recommends clinical examination and blood tests every 3 to 6 months for 5 years, and yearly after that, if clinically indicated (39). Imaging (with CECT) is recommended only if there are signs of disease relapse clinically or biochemically. In stage III and IV DLBCL, in addition to clinical and biochemical examination, CT scan is recommended every 6 months for 2 years, and only if clinically indicated after this period. A decision-analytic Markov model to evaluate the cost-effectiveness of follow-up strategies following first-line immunochemotherapyshowed only minimal survival benefit of routine CT or PET/CT scans when compared with clinical follow-up alone, yet at a significantly higher cost (90). In another study of 680 patients with DLBCL, surveillance imaging detected DLBCL relapse before clinical evaluation inonly 1.6% of patients. There was no difference in survival found in patients detected to have relapse before scheduled follow-up versus at scheduled clinical follow-up, thus not supporting use of routine imaging in surveillance (91). The false-positive rate with PET scans is greater than 20% which can lead to unnecessary further investigations including biopsies and additional cost burden. Also, there is associated patient anxiety and risk of radiation exposure involved. Thus, the existing evidence does notsupport routine surveillance scans, unless clinically indicated (42).
Diffuse large B cell lymphoma is the most common type of NHL and one of the most common malignancies seen in oncology clinics. Functional imaging with FDG-PET/CT provides an in-vivo non-invasive method of assessing disease at the molecular level. The remarkable ongoing advances in precision medicine with development of targeted molecular agents help to facilitate personalized therapeutic selection for each patient. Accurate pretreatment evaluation and response assessment are critical to the optimal management of patients with DLBCL. PET/CT plays a crucial role in management of these patients at various stages, including diagnosis, evaluation of disease burden, response assessment and detection of relapses/recurrence. Other imaging modalities, including US and CT are particularly useful in aiding core biopsies where an excisional biopsy is not possible. MRI has an important role in CNS involvement of disease, as well as emerging as a radiation free alternative to PET/CT, particulary in young and pregnant patients.
Figures and Tables
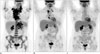 | Fig. 133-year-old woman with DLBCL (PMBL subtype).
A. Maximum intensity projection (MIP) FDG-PET image shows FDG-avid cervical, thoracic, and abdominal lymphadenopathy, as well as diffuse bone marrow uptake. B. Interim MIP FDG-PET image after 2 cycles of chemotherapy shows good metabolic response with residual thoracic lymphadenopathy. C. End of treatment FDG-PET image shows complete response. DLBCL = diffuse large B cell lymphoma, FDG = (F-18)2-fluoro-2-deoxy-D-glucose, PET = positron emission tomography, PMBL = primary mediastinal B-cell lymphoma
|
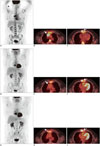 | Fig. 243-year-old man with DLBCL.
A-C. Pre-therapy FDG-PET/CT demonstrates large intensely FDG-avid anterior mediastinal mass (arrow in B), presternal soft tissue mass (arrow in C), and diffuse bone marrow involvement. Focal intense uptake in proximal right upper extremity represents injection site. D-F. Interim FDG-PET/CT, performed after 2 cycles of chemotherapy, demonstrates complete metabolic response in anterior medistinum with residual anterior mediastinal soft tissue (arrow in E); however persistent presternal FDG-avid soft tissue is consistent with incomplete response (arrow in F). G-I. Post-therapy FDG-PET/CT performed after 4 cycles of chemotherapy demonstrates interval increase in size of presternal soft tissue mass (arrow in I), as well as new recurrence in right parasternal region more superiorly (arrow in H). CT = computed tomography, DLBCL = diffuse large B cell lymphoma, FDG = (F-18)2-fluoro-2-deoxy-D-glucose, PET = positron emission tomography
|
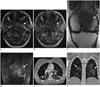 | Fig. 361-year-old woman presented with fullness in her left ear and bilateral neck swelling.Endoscopy revealed left-sided nasopharyngeal mass. Patient also had pain and swelling in left knee.
A. Axial T2-weighted MR image shows mildy hyperintense mass (arrow) involving left nasopharynx. B. Axial contrast-enhanced T1-weighted MR image shows minimal homogeneous enhancement of mass (arrow). C. Coronal T1-weighted MR image shows low signal intensity of tumor relative to fatty marrow of epiphysis (arrow). D. Coronal short tau inversion recovery MR image of knee joint shows irregular hyperintense mass involving distal femoral epiphysis with periosteal reaction and soft tissue (arrow). Pathology demonstrated DLBCL with MYC and BCL-2 overexpression on immunoperoxidase staining, consistent with double hit lymphoma. Patient presented to emergency department after 2 cycles of R-CHOP chemotherapy with cough and shortness of breath. E, F. Axial (E) and coronal (F) contrast-enhanced lung window CT images reveal patchy groundglass opacities in both lower lobes (arrows) suggestive of drug associated pneumonitis. CT = computed tomography, DLBCL = diffuse large B cell lymphoma, R-CHOP = rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone
|
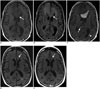 | Fig. 474-year-old man with primary multifocal CNS DLBCL.
A. Axial T1-weighted MR image shows large infiltrative mass involving the genu of corpus callosum and bilateral frontal deep white matter (arrow), which is nearly isointense to white matter. B. On corresponding axial fluid attenuated inversion recovery (FLAIR) MR image, mass is hyperintense to white matter, and there is extensive white matter edema in bilateral frontal lobes. C. Axial contrast-enhanced T1-weighted MR image shows heterogeneous enhancement of mass, as well as involvement of splenium of corpus callosum (arrow) and bilateral periventricular deep white matter (arrowhead). D, E. Post-treatment axial FLAIR (D) and contrast-enhanced T1-weighted MR images show near complete response with marked reduction in size and enhancement of mass (arrows). CNS = central nervous system, DLBCL = diffuse large B cell lymphoma
|
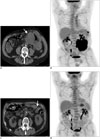 | Fig. 565-year-old man with HIV presented with progressive fatigue and abdominal pain.
A, B. Axial non-enhanced CT (A) and coronal FDG-PET (B) reveal an FDG-avid mass involving small bowel loop, causing aneurysmal dilatation without obstruction (arrow). C, D. Posttherapy axial non-enhanced CT (C) and coronal FDG-PET (D) reveal complete metabolic response with resolution of bowel wall thickening (arrow). CT = computed tomography, FDG = (F-18)2-fluoro-2-deoxy-D-glucose, HIV = human immunodeficiency virus, PET = positron emission tomography
|
 | Fig. 668-year-old man with CLL and transformation to DLBCL.
A. Pre-treatment coronal contrast-enhanced CT image shows massive splenomegaly and enlarged mesenteric lymph nodes (arrow). B. Follow up study after chemotherapy shows treatment response evidenced by interval decrease in size of lymph nodes (arrow) and spleen. C. Axial contrast-enhanced CT image after 6 months reveals new diffuse omental and peritoneal disease (arrows), as well as ascites, in setting of increasing LDH and lymphocytosis. Pathology was consistent with DLBCL. CLL = chronic lymphocytic leukemia, CT = computed tomography, DLBCL = diffuse large B cell lymphoma, LDH = lactate dehydrogenase
|
Table 1
DLBCL Molecular Subtypes

Table 2
Revised Criteria for Response Assessment

References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013; 63:11–30.
2. Møller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation--a population-based study of 1575 cases. Br J Haematol. 2004; 124:151–159.
3. Shenoy PJ, Malik N, Nooka A, Sinha R, Ward KC, Brawley OW, et al. Racial differences in the presentation and outcomes of diffuse large B-cell lymphoma in the United States. Cancer. 2011; 117:2530–2540.
4. Lenz G, Wright GW, Emre NC, Kohlhammer H, Dave SS, Davis RE, et al. Molecular subtypes of diffuse large B-cell lymphoma arise by distinct genetic pathways. Proc Natl Acad Sci U S A. 2008; 105:13520–13525.
5. Iqbal J, Sanger WG, Horsman DE, Rosenwald A, Pickering DL, Dave B, et al. BCL2 translocation defines a unique tumor subset within the germinal center B-cell-like diffuse large B-cell lymphoma. Am J Pathol. 2004; 165:159–166.
6. Basso K, Dalla-Favera R. BCL6: master regulator of the germinal center reaction and key oncogene in B cell lymphomagenesis. Adv Immunol. 2010; 105:193–210.
7. Savage KJ, Johnson NA, Ben-Neriah S, Connors JM, Sehn LH, Farinha P, et al. MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy. Blood. 2009; 114:3533–3537.
8. Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001; 194:1861–1874.
9. Lenz G, Davis RE, Ngo VN, Lam L, George TC, Wright GW, et al. Oncogenic CARD11 mutations in human diffuse large B cell lymphoma. Science. 2008; 319:1676–1679.
10. Green MR, Monti S, Rodig SJ, Juszczynski P, Currie T, O'Donnell E, et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD-1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood. 2010; 116:3268–3277.
11. Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003; 198:851–862.
12. Cazals-Hatem D, Lepage E, Brice P, Ferrant A, d'Agay MF, Baumelou E, et al. Primary mediastinal large B-cell lymphoma. A clinicopathologic study of 141 cases compared with 916 nonmediastinal large B-cell lymphomas, a GELA (“Groupe d'Etude des Lymphomes de l’Adulte“) study. Am J Surg Pathol. 1996; 20:877–888.
13. Dunleavy K, Grant C, Eberle FC, Pittaluga S, Jaffe ES, Wilson WH. Gray zone lymphoma: better treated like Hodgkin lymphoma or mediastinal large B-cell lymphoma? Curr Hematol Malig Rep. 2012; 7:241–247.
14. Aukema SM, Siebert R, Schuuring E, van Imhoff GW, Kluin-Nelemans HC, Boerma EJ, et al. Double-hit B-cell lymphomas. Blood. 2011; 117:2319–2331.
15. Fisher RI, Gaynor ER, Dahlberg S, Oken MM, Grogan TM, Mize EM, et al. Comparison of a standard regimen (CHOP) with three intensive chemotherapy regimens for advanced non-Hodgkin's lymphoma. N Engl J Med. 1993; 328:1002–1006.
16. Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002; 346:235–242.
17. Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood. 2010; 116:2040–2045.
18. Batlevi CL, Matsuki E, Brentjens RJ, Younes A. Novel immunotherapies in lymphoid malignancies. Nat Rev Clin Oncol. 2016; 13:25–40.
19. Viardot A, Goebeler ME, Hess G, Neumann S, Pfreundschuh M, Adrian N, et al. Phase 2 study of the bispecific T-cell engager (BiTE) antibody blinatumomab in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2016; 127:1410–1416.
20. Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015; 33:540–549.
21. Sanford M. Blinatumomab: first global approval. Drugs. 2015; 75:321–327.
22. Topp MS, Gökbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015; 16:57–66.
23. Buie LW, Pecoraro JJ, Horvat TZ, Daley RJ. Blinatumomab: a first-in-class bispecific T-cell engager for precursor B-cell acute lymphoblastic leukemia. Ann Pharmacother. 2015; 49:1057–1067.
24. Goebeler ME, Knop S, Viardot A, Kufer P, Topp MS, Einsele H, et al. Bispecific T-cell engager (BiTE) antibody construct blinatumomab for the treatment of patients with relapsed/refractory non-Hodgkin lymphoma: final results from a phase I study. J Clin Oncol. 2016; 34:1104–1111.
25. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012; 12:252–264.
26. Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011; 364:2517–2526.
27. Ansell SM, Lesokhin AM, Borrello I, Halwani A, Scott EC, Gutierrez M, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med. 2015; 372:311–319.
28. Armand P, Nagler A, Weller EA, Devine SM, Avigan DE, Chen YB, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: results of an international phase II trial. J Clin Oncol. 2013; 31:4199–4206.
29. Friedberg JW. Relapsed/refractory diffuse large B-cell lymphoma. Hematology Am Soc Hematol Educ Program. 2011; 2011:498–505.
30. Raut LS, Chakrabarti PP. Management of relapsed-refractory diffuse large B cell lymphoma. South Asian J Cancer. 2014; 3:66–70.
31. Ahuja AT, Ying M, Ho SY, Antonio G, Lee YP, King AD, et al. Ultrasound of malignant cervical lymph nodes. Cancer Imaging. 2008; 8:48–56.
32. Ahuja AT, Ying M, Yuen HY, Metreweli C. ‘Pseudocystic’ appearance of non-Hodgkin's lymphomatous nodes: an infrequent finding with high-resolution transducers. Clin Radiol. 2001; 56:111–115.
33. McInnes MD, Kielar AZ, Macdonald DB. Percutaneous image-guided biopsy of the spleen: systematic review and meta-analysis of the complication rate and diagnostic accuracy. Radiology. 2011; 260:699–708.
34. Burke C, Thomas R, Inglis C, Baldwin A, Ramesar K, Grace R, et al. Ultrasound-guided core biopsy in the diagnosis of lymphoma of the head and neck. A 9 year experience. Br J Radiol. 2011; 84:727–732.
35. Larcos G, Farlow DC, Antico VF, Gruenewald SM, Boyages J. The role of high dose 67-gallium scintigraphy in staging untreated patients with lymphoma. Aust N Z J Med. 1994; 24:5–8.
36. Israel O, Front D, Epelbaum R, Ben-Haim S, Jerushalmi J, Kleinhaus U, et al. Residual mass and negative gallium scintigraphy in treated lymphoma. J Nucl Med. 1990; 31:365–368.
37. Front D, Bar-Shalom R, Epelbaum R, Haim N, Ben-Arush MW, Ben-Shahar M, et al. Early detection of lymphoma recurrence with gallium-67 scintigraphy. J Nucl Med. 1993; 34:2101–2104.
38. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32:3059–3068.
39. NCCN. Non-Hodgkin's Lymphomas. Washington, DC: NCCN;2016.
40. Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, et al. An enhanced International Prognostic Index (NCCN-IPI) for patients with diffuse large B-cell lymphoma treated in the rituximab era. Blood. 2014; 123:837–842.
41. Cheson BD. Staging and response assessment in lymphomas: the new Lugano classification. Chin Clin Oncol. 2015; 4:5.
42. Cheson BD. Role of functional imaging in the management of lymphoma. J Clin Oncol. 2011; 29:1844–1854.
43. Vriens D, Visser EP, de Geus-Oei LF, Oyen WJ. Methodological considerations in quantification of oncological FDG PET studies. Eur J Nucl Med Mol Imaging. 2010; 37:1408–1425.
44. Kostakoglu L, Cheson BD. State-of-the-art research on “Lymphomas: role of molecular imaging for staging, prognostic evaluation, and treatment response”. Front Oncol. 2013; 3:212.
45. Berthet L, Cochet A, Kanoun S, Berriolo-Riedinger A, Humbert O, Toubeau M, et al. In newly diagnosed diffuse large B-cell lymphoma, determination of bone marrow involvement with 18F-FDG PET/CT provides better diagnostic performance and prognostic stratification than does biopsy. J Nucl Med. 2013; 54:1244–1250.
46. Adams HJ, Kwee TC, Fijnheer R, Dubois SV, Nievelstein RA, de Klerk JM, et al. Bone marrow 18F-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography cannot replace bone marrow biopsy in diffuse large B-cell lymphoma. Am J Hematol. 2014; 89:726–731.
47. Cerci JJ, Györke T, Fanti S, Paez D, Meneghetti JC, Redondo F, et al. Combined PET and biopsy evidence of marrow involvement improves prognostic prediction in diffuse large B-cell lymphoma. J Nucl Med. 2014; 55:1591–1597.
48. Juweid ME, Stroobants S, Hoekstra OS, Mottaghy FM, Dietlein M, Guermazi A, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. J Clin Oncol. 2007; 25:571–578.
49. Meignan M, Gallamini A, Meignan M, Gallamini A, Haioun C. Report on the first international workshop on interim-PET-scan in lymphoma. Leuk Lymphoma. 2009; 50:1257–1260.
50. Itti E, Meignan M, Berriolo-Riedinger A, Biggi A, Cashen AF, Véra P, et al. An international confirmatory study of the prognostic value of early PET/CT in diffuse large B-cell lymphoma: comparison between Deauville criteria and ΔSUVmax. Eur J Nucl Med Mol Imaging. 2013; 40:1312–1320.
51. Carr R, Fanti S, Paez D, Cerci J, Györke T, Redondo F, et al. Prospective international cohort study demonstrates inability of interim PET to predict treatment failure in diffuse large B-cell lymphoma. J Nucl Med. 2014; 55:1936–1944.
52. Dührsen U, Hüttmann A, Jöckel KH, Müller S. Positron emission tomography guided therapy of aggressive non-Hodgkin lymphomas--the PETAL trial. Leuk Lymphoma. 2009; 50:1757–1760.
53. van der Kolk LE, Grillo-López AJ, Baars JW, Hack CE, van Oers MH. Complement activation plays a key role in the side-effects of rituximab treatment. Br J Haematol. 2001; 115:807–811.
54. Hadjinicolaou AV, Nisar MK, Parfrey H, Chilvers ER, Ostör AJ. Non-infectious pulmonary toxicity of rituximab: a systematic review. Rheumatology (Oxford). 2012; 51:653–662.
55. Adams HJ, Nievelstein RA, Kwee TC. Prognostic value of complete remission status at end-of-treatment FDG-PET in R-CHOP-treated diffuse large B-cell lymphoma: systematic review and meta-analysis. Br J Haematol. 2015; 170:185–191.
56. Coughlan M, Elstrom R. The use of FDG-PET in diffuse large B cell lymphoma (DLBCL): predicting outcome following first line therapy. Cancer Imaging. 2014; 14:34.
57. Cremerius U, Fabry U, Wildberger JE, Zimny M, Reinartz P, Nowak B, et al. Pre-transplant positron emission tomography (PET) using fluorine-18-fluoro-deoxyglucose (FDG) predicts outcome in patients treated with high-dose chemotherapy and autologous stem cell transplantation for non-Hodgkin's lymphoma. Bone Marrow Transplant. 2002; 30:103–111.
58. Sauter CS, Matasar MJ, Meikle J, Schoder H, Ulaner GA, Migliacci JC, et al. Prognostic value of FDG-PET prior to autologous stem cell transplantation for relapsed and refractory diffuse large B-cell lymphoma. Blood. 2015; 125:2579–2581.
59. Brastianos PK, Batchelor TT. Primary central nervous system lymphoma: overview of current treatment strategies. Hematol Oncol Clin North Am. 2012; 26:897–916.
60. Korfel A, Schlegel U. Diagnosis and treatment of primary CNS lymphoma. Nat Rev Neurol. 2013; 9:317–327.
61. Mohile NA, Abrey LE. Primary central nervous system lymphoma. Neurol Clin. 2007; 25:1193–1207. xi
62. Grisariu S, Avni B, Batchelor TT, van den Bent MJ, Bokstein F, Schiff D, et al. Neurolymphomatosis: an International Primary CNS Lymphoma Collaborative Group report. Blood. 2010; 115:5005–5011.
63. Bollen EL, Brouwer RE, Hamers S, Hermans J, Kluin M, Sankatsing SU, et al. Central nervous system relapse in non-Hodgkin lymphoma. A single-center study of 532 patients. Arch Neurol. 1997; 54:854–859.
64. Mohile NA, Deangelis LM, Abrey LE. The utility of body FDG PET in staging primary central nervous system lymphoma. Neuro Oncol. 2008; 10:223–228.
65. Mayerhoefer ME, Karanikas G, Kletter K, Prosch H, Kiesewetter B, Skrabs C, et al. Evaluation of diffusion-weighted MRI for pretherapeutic assessment and staging of lymphoma: results of a prospective study in 140 patients. Clin Cancer Res. 2014; 20:2984–2993.
66. Littooij AS, Kwee TC, Barber I, Granata C, Vermoolen MA, Enríquez G, et al. Whole-body MRI for initial staging of paediatric lymphoma: prospective comparison to an FDG-PET/CT-based reference standard. Eur Radiol. 2014; 24:1153–1165.
67. Heacock L, Weissbrot J, Raad R, Campbell N, Friedman KP, Ponzo F, et al. PET/MRI for the evaluation of patients with lymphoma: initial observations. AJR Am J Roentgenol. 2015; 204:842–848.
68. Herrmann K, Queiroz M, Huellner MW, de Galiza Barbosa F, Buck A, Schaefer N, et al. Diagnostic performance of FDG-PET/MRI and WB-DW-MRI in the evaluation of lymphoma: a prospective comparison to standard FDG-PET/CT. BMC Cancer. 2015; 15:1002.
69. Riedel DJ, Rositch AF, Redfield RR, Blattner WA. HIV-associated lymphoma sub-type distribution, immunophenotypes and survival in an urban clinic population. Leuk Lymphoma. 2016; 57:306–312.
70. Agarwal PA, Menon S, Smruti BK, Singhal BS. Primary central nervous system lymphoma: a profile of 26 cases from Western India. Neurol India. 2009; 57:756–763.
71. Bayraktar S, Bayraktar UD, Ramos JC, Stefanovic A, Lossos IS. Primary CNS lymphoma in HIV positive and negative patients: comparison of clinical characteristics, outcome and prognostic factors. J Neurooncol. 2011; 101:257–265.
72. Johnson BA, Fram EK, Johnson PC, Jacobowitz R. The variable MR appearance of primary lymphoma of the central nervous system: comparison with histopathologic features. AJNR Am J Neuroradiol. 1997; 18:563–572.
73. Hare SS, Souza CA, Bain G, Seely JM, Frcpc , Gomes MM, et al. The radiological spectrum of pulmonary lymphoproliferative disease. Br J Radiol. 2012; 85:848–864.
74. Anis M, Irshad A. Imaging of abdominal lymphoma. Radiol Clin North Am. 2008; 46:265–285. viii–ix.
75. Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC, Lawhorn-Crews JM, et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med. 1998; 4:1334–1336.
76. Leng K. Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. 3'-Deoxy-3'-[18F]fluorothymidine. Accessed January 15, 2015. http://www.ncbi.nlm.nih.gov/books/NBK23373/.
77. Sherley JL, Kelly TJ. Regulation of human thymidine kinase during the cell cycle. J Biol Chem. 1988; 263:8350–8358.
78. Tian J, Yang X, Yu L, Chen P, Xin J, Ma L, et al. A multicenter clinical trial on the diagnostic value of dual-tracer PET/CT in pulmonary lesions using 3'-deoxy-3'-18F-fluorothymidine and 18F-FDG. J Nucl Med. 2008; 49:186–194.
79. Zhao S, Kuge Y, Kohanawa M, Takahashi T, Zhao Y, Yi M, et al. Usefulness of 11C-methionine for differentiating tumors from granulomas in experimental rat models: a comparison with 18F-FDG and 18F-FLT. J Nucl Med. 2008; 49:135–141.
80. Troost EG, Vogel WV, Merkx MA, Slootweg PJ, Marres HA, Peeters WJ, et al. 18F-FLT PET does not discriminate between reactive and metastatic lymph nodes in primary head and neck cancer patients. J Nucl Med. 2007; 48:726–735.
81. Tadmor T, Shvidel L, Bairey O, Goldschmidt N, Ruchlemer R, Fineman R, et al. Richter's transformation to diffuse large B-cell lymphoma: a retrospective study reporting clinical data, outcome, and the benefit of adding rituximab to chemotherapy, from the Israeli CLL Study Group. Am J Hematol. 2014; 89:E218–E222.
82. Giardino AA, O'Regan K, Jagannathan JP, Elco C, Ramaiya N, Lacasce A. Richter's transformation of chronic lymphocytic leukemia. J Clin Oncol. 2011; 29:e274–e276.
83. Bruzzi JF, Macapinlac H, Tsimberidou AM, Truong MT, Keating MJ, Marom EM, et al. Detection of Richter's transformation of chronic lymphocytic leukemia by PET/CT. J Nucl Med. 2006; 47:1267–1273.
84. Falchi L, Keating MJ, Marom EM, Truong MT, Schlette EJ, Sargent RL, et al. Correlation between FDG/PET, histology, characteristics, and survival in 332 patients with chronic lymphoid leukemia. Blood. 2014; 123:2783–2790.
85. Jaffe ES. The 2008 WHO classification of lymphomas: implications for clinical practice and translational research. Hematology Am Soc Hematol Educ Program. 2009; 523–531.
86. Zuckerman D, Seliem R, Hochberg E. Intravascular lymphoma: the oncologist's “great imitator”. Oncologist. 2006; 11:496–502.
87. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissue. 4th ed. Lyon: IARC;2008.
88. Shimada K, Kinoshita T, Naoe T, Nakamura S. Presentation and management of intravascular large B-cell lymphoma. Lancet Oncol. 2009; 10:895–902.
89. Yamamoto A, Kikuchi Y, Homma K, O'uchi T, Furui S. Characteristics of intravascular large B-cell lymphoma on cerebral MR imaging. AJNR Am J Neuroradiol. 2012; 33:292–296.
90. Huntington SF, Svoboda J, Doshi JA. Cost-effectiveness analysis of routine surveillance imaging of patients with diffuse large B-cell lymphoma in first remission. J Clin Oncol. 2015; 33:1467–1474.
91. Thompson CA, Ghesquieres H, Maurer MJ, Cerhan JR, Biron P, Ansell SM, et al. Utility of routine post-therapy surveillance imaging in diffuse large B-cell lymphoma. J Clin Oncol. 2014; 32:3506–3512.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download