Abstract
Objective
This study aimed to assess the technical feasibility, procedural safety, and long-term therapeutic efficacy of a small-sized ambulatory thoracic vent (TV) device for the treatment of pneumothorax.
Materials and Methods
From November 2012 to July 2013, 18 consecutive patients (3 females, 15 males) aged 16–64 years (mean: 34.7 ± 14.9 years, median: 29 years) were enrolled prospectively. Of these, 15 patients had spontaneous pneumothorax and 3 had iatrogenic pneumothorax. A Tru-Close TV with a small-bore (11- or 13-Fr) catheter was inserted under bi-plane fluoroscopic assistance.
Results
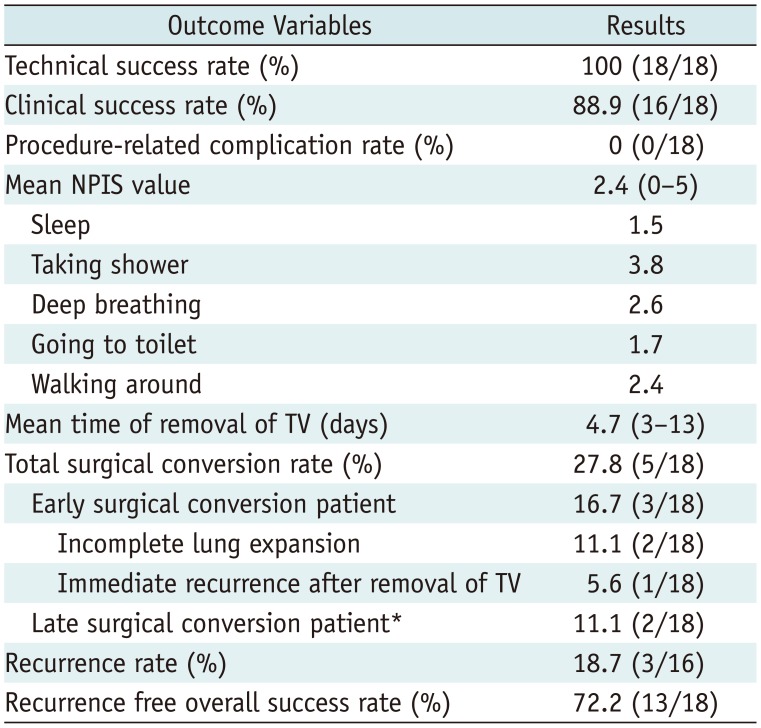
Technical success was achieved in all patients. Complete lung re-expansion was achieved at 24 hours in 88.9% of patients (16/18 patients). All patients tolerated the procedure and no major complications occurred. The patients' mean numeric pain intensity score was 2.4 (range: 0–5) in daily life activity during the TV treatment. All patients with spontaneous pneumothorax underwent outpatient follow-up. The mean time to TV removal was 4.7 (3–13) days. Early surgical conversion rate of 16.7% (3/18 patients) occurred in 2 patients with incomplete lung expansion and 1 patient with immediate pneumothorax recurrence post-TV removal; and late surgical conversion occurred in 2 of 18 patients (11.1%). The recurrence-free long-term success rate was 72.2% (13/18 patients) during a 3-year follow-up period from November 2012 to June 2016.
Pneumothorax is a frequently occurring clinical problem with a reported incidence of 18–28/100000 cases per year in men and 1.2–6/100000 per year in women (1).
Pneumothorax is classified as either spontaneous or iatrogenic. Spontaneous pneumothorax can be further classified into primary cases, which involve healthy individuals without underlying lung disease, or secondary cases, which involve patients with underlying lung diseases such as pulmonary infection, emphysema, and malignancy. Traditional guidelines for the primary treatment of pneumothorax differ and include simple aspiration, small-bore catheter (≤ 14 Fr) or chest tube (16–22 Fr) drainage, and ambulatory management with a small-bore catheter connected to Heimlich flutter valves (23). Treatment via conventional chest tube drainage connected to a suction system is usually associated with hospitalization, immobilization, and discomfort. However, reliable patients who do not want to be hospitalized can be treated in an outpatient department with a small-bore catheter attached to a Heimlich valve after lung reexpansion (2).
The thoracic vent (TV), a small-sized portable pneumothorax kit, is a minimally invasive, immediate salvage device for the treatment of pneumothorax that has been used successfully in several hospitals (456). With this device, immobilization and hospitalization are unnecessary because there is no need to connect the TV to an underwater seal device. Some studies, including a randomized comparison study by Röggla et al. (5), have assessed the effectiveness and advantage of TV vs. conventional tube drainage (67). To date, however, few reports have addressed long-term recurrence rates with TV treatment.
This prospective study aimed to assess the technical feasibility, procedural safety, and long-term therapeutic efficacy of TV for the treatment of pneumothorax.
The Institutional Review Board approved this prospective study (IRB approval number 3-2012-0114), and informed consent was obtained from all patients. Patients who presented with spontaneous or iatrogenic pneumothorax from November 2012 to July 2013 were enrolled.
The exclusion criteria for the TV treatment were as follows: 1) hydropneumothorax, 2) hemopneumothorax, 3) patient refusal, and 4) underlying pulmonary disease requiring supplemental oxygen.
A total of 18 patients (3 females, 15 males) between the ages of 16 and 64 years (mean: 34.7 ± 14.9 years, median: 29 years) were enrolled. The mean time to intervention from the development of symptoms of pneumothorax was 2.3 days (range: 1–6 days). The baseline characteristics of the patients are shown in Table 1. Fifteen patients had spontaneous pneumothorax (primary = 7, secondary = 8). The remaining 3 patients had iatrogenic pneumothorax (2 cases of subclavian vein catheterization, and transthoracic needle biopsy).
Pneumothorax size was quantified using the equation reported by Collins et al. (8): pneumothorax size (%) = 4.2 + (4.7 × [A + B + C]), where A is the lung apex to apex, B is the upper half from the midpoint, and C is the lower half from the midpoint.
Additionally, all patients participated in a personal interview about their experiences of pain at different activity levels of daily life (i.e., sleep, showering, deep breathing, going to the toilet, walking around) during TV treatment according to a numeric pain intensity scale (NPIS; range: 0–10).
We used the Tru-Close® TV (UreSil, Skokie, IL, USA) (Fig. 1), which comprises a flexible polyurethane catheter connected to a plastic chamber containing a 1-way valve. Positive pressure within the pleural space is indicated by a pressure-sensitive diaphragm contained within the plastic chamber (46). TVs with small-bore (11- or 13-Fr) catheters were inserted under fluoroscopic guidance.
For traditional fluoroscopic-interventional device insertion, we used bi-plane fluoroscopy to ensure a safe and rapid procedure (Fig. 2). With the patient resting in the supine position, the puncture point was determined under bi-plane fluoroscopic guidance. After administering local anesthesia (2% lidocaine), the pneumothorax cavity was punctured with a 21-gauge Chiba needle (A&A M.D., Seongnam, Korea), usually into the second to fourth intercostal space along the midclavicular line. After confirming proper needle puncture within the pleural space under bi-plane fluoroscopy, a 0.018-inch hair-wire (slim wire, A&A M.D.) and yellow sheath (A&A M.D.) were introduced sequentially. The guide wire was then exchanged for a 0.035-inch, 80-cm-long stiff guide wire (Radifocus Guidewire; Terumo, Tokyo, Japan), and the TV device was finally inserted over-the-wire using a 7-Fr dilator.
Immediately after insertion of the TV, movement of the red-colored diaphragm indicated drainage of the pneumothorax based on the gradient between the intrathoracic and extracorporeal pressures. If the diaphragm did not move, the patient was encouraged to breathe deeply to enhance intrathoracic pressure, thus allowing the diaphragm movement; or diaphragm movement would be initiated by triggering pneumothorax aspiration with a 20-mL syringe.
Movement of the red-colored diaphragm stopped upon full expansion of the affected lung. Subsequently, a chest radiography evaluation was performed under full expiration status. If full expansion of the involved lung was observed on a chest radiograph, an occlusion plug was applied to evaluate further air leakage during follow-up chest radiography on the next day. Removal of the entire device was recommended if follow-up chest radiography indicated all negative signs on the occlusion plug test and a fully expanded lung without pneumothorax recurrence.
Technical success was defined as successful placement of the TV within the pleural cavity without procedure-related complications. Clinical success after TV insertion was defined as a complete resolution of baseline clinical symptoms and/or signs related to pneumothorax. Chest radiographs were taken immediately after TV insertion and daily thereafter to assess re-expansion of the affected lung. Clinically stable patients were discharged from the hospital with the TV in place. Oral medications (analgesics, non-steroid anti-inflammatory drug, and anti-acid) were prescribed and the patients were followed-up at an outpatient clinic.
All enrolled patients underwent follow-up for a mean duration of 36.4 months (range: 19–42 months) from November 2012 to June 2016. Kaplan-Meier analysis was used to estimate the probability of being recurrence-free in the patients with TV insertion.
Table 2 summarizes the results of TV insertion in this study, with a mean follow-up duration of 36.4 months (range: 19–42 months).
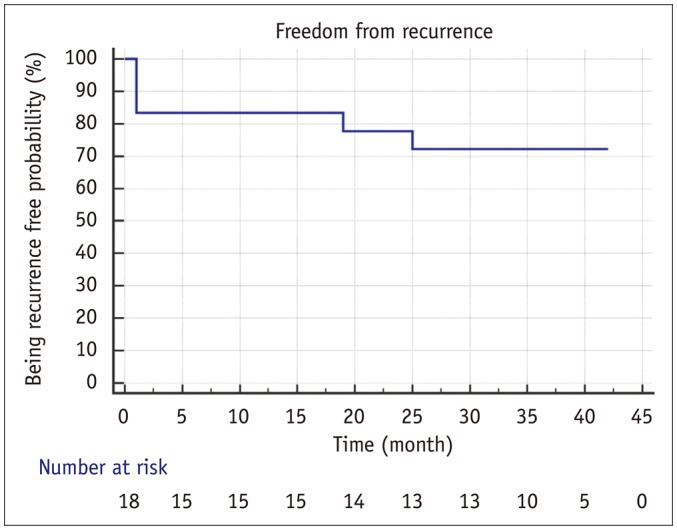
Thoracic vent insertion was technically successful without complications in all patients. Clinical success (i.e., complete re-expansion of the affected lung) was achieved in 88.9% (16/18) of cases at 24 hours. The degree of pain at different activity levels is shown in Table 2; and the mean NPIS during TV treatment was 2.4 (range: 0–5). All patients with spontaneous pneumothorax were followed-up on an outpatient basis. The mean time to TV removal was 4.7 days (range: 3–13 days). Of the 18 patients, 2 were converted to video-assisted thoracoscopic (VATS) wedge resection of the affected lung because of incomplete lung expansion at 5 days after TV insertion (treatment failure), and 1 underwent VATS wedge resection and pleurodesis because of an immediate recurrence at 17 days after TV removal (early recurrence). During follow-up of the remaining 15 patients, 2 experienced pneumothorax recurrence at 19 and 25 months after TV removal, respectively (late recurrence). The former patient underwent VATS wedge resection for multiple bullae, and the latter was treated via tube thoracotomy. The latter patient suffered from multiple cystic lung disease and was managed by repeated tube thoracotomy instead of surgery. Kaplan-Meier analysis of freedom from recurrence for patients with TV insertion is shown in Figure 3. The recurrence-free probability of pneumothorax at 1, 2, and 3 years was 83.3, 77.8, and 72.2%, respectively. The mean freedom from recurrent pneumothorax was 32.9 months (95% confidence interval, 25.7–40.1 months).
In some hospitals, needle aspiration is used to treat small pneumothorax on an outpatient basis. Although needle aspiration is a simple procedure, it is difficult to assess whether there is a persistent air leak after needle aspiration. Therefore, minimally invasive chest tube drainage is considered more clinically useful in treating patients with a large pneumothorax. Treatment of pneumothorax with a valved device is not a new concept. Given the advantages of mobility and outpatient ambulatory management, the well known Heimlich valve has been used in several hospitals (9). However, small-bore catheters attached to flutter valves have reported problems such as difficulty of air leak estimation (i.e., caregivers should disconnect the drain and connect it to an underwater drain to see if there is any air leak), possibility of the valve falling off the tube or valve dislodgement, and catheter kinking (41011). As a modality for the ambulatory management of spontaneous pneumothorax, TV was designed to overcome problems associated with the small-bore catheter system. Several recent studies have shown the safety and efficacy of a minimally invasive approach involving TV (45612131415). Since monitoring for air leaks is possible, TV can be removed after the confirmation of pneumothorax resolution. Furthermore, since TV uses a one-way valve within a plastic chamber that is permanently attached to a catheter, it is not associated with the problem of valve disconnection. Under these circumstances, we conducted this study to assess the effectiveness and long-term therapeutic efficacy of TV treatment.
In this article, we found that TV is not only safe and effective for the immediate treatment of pneumothorax, but also provides a high long-term recurrence-free success rate. The rate of incomplete expansion was very low (11.1%, 2 of 18 patients), and only 1 patient underwent surgery after TV removal because of an immediate recurrence. The former two patients had a very large amount of pneumothorax (99.9 and 73.4%, respectively) with subpleural fibrosis and bullae, and the latter patient had a moderate amount of pneumothorax (39.2%) with several subpleural bullae. The 3-year follow-up of patients treated with TV in this prospective study revealed a high recurrence-free overall success rate (72.2%, 13 of 18 patients). In the patients with late surgical coversion, initial pneumothorax was also successfully treated with TV, but rupture of another cystic lesion or bulla resulted in recurrent pneumothorax.
In our patients, hospitalization on the day of TV insertion was avoided, and early ambulation was possible. Most of those patients reported little pain associated with the TV device and could lead a normal daily life, including sleep, taking a shower, going to the toilet, and walking around. On the NPIS (0–10), the mean value was 2.4 (range: 0–5) for different activity levels in daily life. Thus, patients with an inserted TV can receive treatment on an outpatient basis and are not required to stop working or attending school. A prospective trial to compare the TV treatment with other treatment options is needed; however, the consequent advantage of outpatient TV treatment can reduce medical expense and provide economic benefits to patients (6).
We achieved a high technical success rate without complication (100%, 18 of 18 patients) because the procedure was performed under bi-plane fluoroscopic guidance to minimize the risk of deep penetration of the puncture needle. Moreover, the procedure is relatively simple to perform, as compared to chest tube insertion or surgery; therefore, it is less burdensome for patients, as well as physicians. Several TV-associated complications have been reported, including device-occlusion caused by clots or sludges, thoracic wall hematoma caused by malpuncture or repeated puncture, hemothorax caused by unintended arterial laceration, and bronchopleural fistula caused by misuse in a patient with pleural adhesion-associated pneumothorax (451617); however, no major complications were observed in our study.
Röggla et al. (5) conducted a randomized study to compare TV treatment with intercostal tube drainage in the management of pneumothorax. They suggested that TV is as effective as intercostal tube drainage, with no significant differences in the rate of reexpansion and complications. Furthermore, the TV patient group reported less pain than the intercostal tube drainage group. Majority of the TV patient group were treated on an outpatient basis. These authors accordingly recommended TV as the first-line treatment of choice for pneumothorax. However, follow-up data or recurrence rate according to treatment was not evaluated. Recurrence rates of 7–24% are reported for patients treated by small-bore catheter with Heimlich valve system (18). In our study, the 3-year follow-up recurrence rate of TV treatment was 18.7% (3/16 patients), which was not significantly different from the recurrence rates of small-bore catheter system. This result indicated that TV treatment is as effective in terms of recurrence as the small-bore catheter system.
Unfortunately, this study suffers from the following limitations. First, the study population was relatively small and the study was conducted in a single center. Second, we did not directly compare the results of our study with those of other treatment methods such as simple needle aspiration, intercostal chest tube drainage, small-bore catheter system, and VATS. However, we performed a prospective investigation of this procedure with a long-term follow up period. The outcomes of this study therefore provide fairly effective clinical results. Further randomized comparison trials with a larger population group are required to confirm the current results.
In conclusion, the use of a small-bore ambulatory TV can be indicated for the outpatient management of pneumothorax. This device could also be a useful primary treatment for spontaneous or iatrogenic pneumothorax with an acceptable long-term recurrence-free rate.
References
1. Melton LJ 3rd, Hepper NG, Offord KP. Incidence of spontaneous pneumothorax in Olmsted County, Minnesota: 1950 to 1974. Am Rev Respir Dis. 1979; 120:1379–1382. PMID: 517861.
2. Baumann MH, Strange C, Heffner JE, Light R, Kirby TJ, Klein J, et al. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001; 119:590–602. PMID: 11171742.
3. Henry M, Arnold T, Harvey J. Pleural Diseases Group. Standards of Care Committee. British Thoracic Society. BTS guidelines for the management of spontaneous pneumothorax. Thorax. 2003; 58(Suppl 2):ii39–ii52. PMID: 12728149.

4. Dernevik L, Roberts D, Hamraz B, Nordstrand-Myntevik M. Management of pneumothorax with a mini-drain in ambulatory and hospitalized patients. Scand Cardiovasc J. 2003; 37:172–176. PMID: 12881149.

5. Röggla M, Wagner A, Brunner C, Röggla G. The management of pneumothorax with the thoracic vent versus conventional intercostal tube drainage. Wien Klin Wochenschr. 1996; 108:330–333. PMID: 8767986.
6. Tsuchiya T, Sano A. Outpatient treatment of pneumothorax with a thoracic vent: economic benefit. Respiration. 2015; 90:33–39. PMID: 25997413.

7. Samelson SL, Goldberg EM, Ferguson MK. The thoracic vent. Clinical experience with a new device for treating simple pneumothorax. Chest. 1991; 100:880–882. PMID: 1889296.
8. Collins CD, Lopez A, Mathie A, Wood V, Jackson JE, Roddie ME. Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis based on volume measurements from helical CT. AJR Am J Roentgenol. 1995; 165:1127–1130. PMID: 7572489.

10. Mariani PJ, Sharma S. Iatrogenic tension pneumothorax complicating outpatient Heimlich valve chest drainage. J Emerg Med. 1994; 12:477–479. PMID: 7963393.

11. Crocker HL, Ruffin RE. Patient-induced complications of a Heimlich flutter valve. Chest. 1998; 113:838–839. PMID: 9515868.

12. Kobayashi Y. [Two cases of pneumothorax using the thoracic vent (TV) on an outpatient basis]. Nihon Kokyuki Gakkai Zasshi. 2001; 39:256–259. PMID: 11481824.
13. Sano A, Tsuchiya T, Nagano M. Outpatient drainage therapy with a thoracic vent for traumatic pneumothorax due to bull attack. Korean J Thorac Cardiovasc Surg. 2014; 47:563–565. PMID: 25551083.

14. Sato N, Abe K, Ishibashi N, Imai T. [Effectiveness of portable thoracic drainage kit for outpatient treatment of spontaneous pneumothorax]. Kyobu Geka. 2016; 69:418–422. PMID: 27246123.
15. Yotsumoto T, Sano A, Sato Y. [Spontaneous pneumothorax during pregnancy successfully managed with a thoracic vent before surgical therapy; report of a case]. Kyobu Geka. 2015; 68:1031–1033. PMID: 26555922.
16. Martin T, Fontana G, Olak J, Ferguson M. Use of pleural catheter for the management of simple pneumothorax. Chest. 1996; 110:1169–1172. PMID: 8915215.
17. Jones AE, Knoepp LF, Oxley DD. Bronchopleural fistula resulting from the use of a thoracic vent: a case report and review. Chest. 1998; 114:1781–1784. PMID: 9872222.
18. Brims FJ, Maskell NA. Ambulatory treatment in the management of pneumothorax: a systematic review of the literature. Thorax. 2013; 68:664–669. PMID: 23515437.

Fig. 1
Thoracic vent.
External dimensions of thoracic vent are 9.5 (length) × 2.5 (width) × 2 cm (height). Thoracic vent comprises flexible urethane catheter with outer diameter of 11-or 13-Fr and length of 10–13 cm and removable in-line trocar connected to 1-way valve. Red-colored diaphragm movement represents pneumothorax drainage according to gradient between intrathoracic and extracorporeal pressures.

Fig. 2
Thoracic vent insertion under fluoroscopic guidance.
A. 21-gauge Chiba needle (arrow) is inserted into second to fourth intercostal space in midclavicular line. Following confirmation of needle position within pleural space under bi-plane fluoroscopic guidance, 0.018-inch hair-wire (arrowhead) and yellow sheath are introduced sequentially. B, C. Guide wire is then exchanged for 0.035-inch radiofocus stiff wire (arrow), and thoracic vent device is inserted over-wire with 7-Fr dilator.
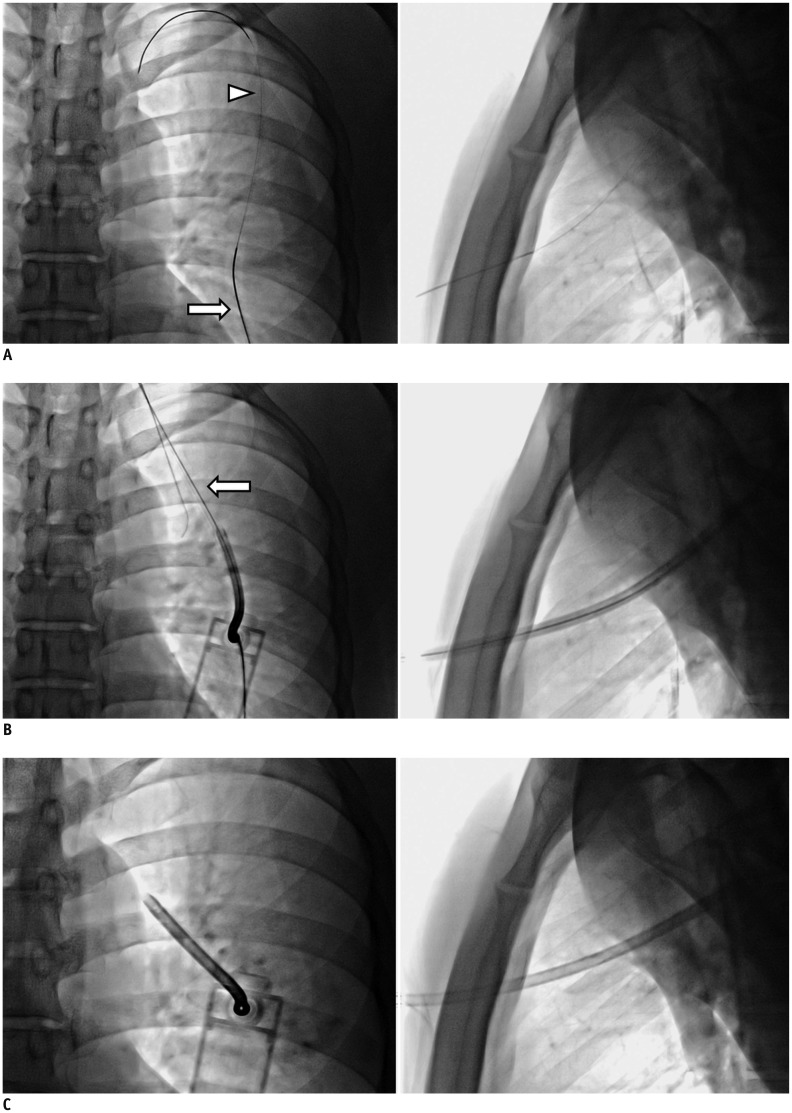
Table 1
Baseline Characteristics of Patients
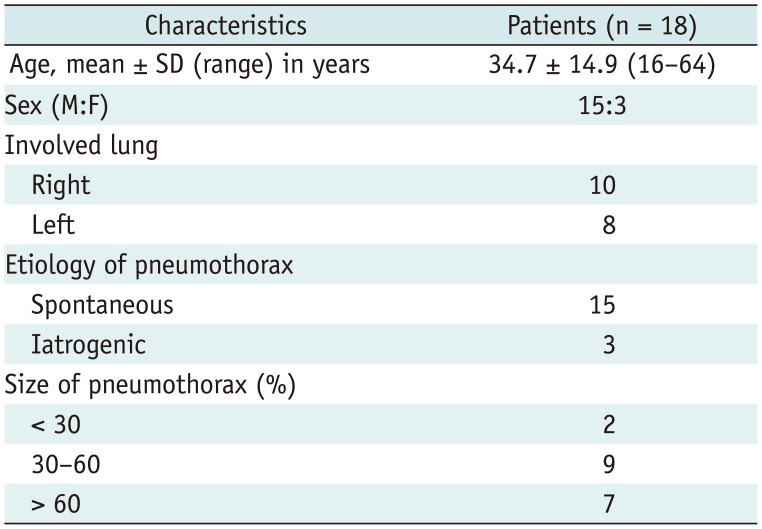
Table 2
Outcomes of TV insertion (n = 18)




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download