1. Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011; 364:226–235. PMID:
21247313.

2. Glaser R, Selzer F, Faxon DP, Laskey WK, Cohen HA, Slater J, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation. 2005; 111:143–149. PMID:
15623544.

3. Narula J, Kovacic JC. Putting TCFA in clinical perspective. J Am Coll Cardiol. 2014; 64:681–683. PMID:
25125299.

4. Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007; 50:319–326. PMID:
17659199.

5. Motoyama S, Sarai M, Harigaya H, Anno H, Inoue K, Hara T, et al. Computed tomographic angiography characteristics of atherosclerotic plaques subsequently resulting in acute coronary syndrome. J Am Coll Cardiol. 2009; 54:49–57. PMID:
19555840.

6. Inoue K, Motoyama S, Sarai M, Sato T, Harigaya H, Hara T, et al. Serial coronary CT angiography-verified changes in plaque characteristics as an end point: evaluation of effect of statin intervention. JACC Cardiovasc Imaging. 2010; 3:691–698. PMID:
20633846.
7. Motoyama S, Sarai M, Narula J, Ozaki Y. Coronary CT angiography and high-risk plaque morphology. Cardiovasc Interv Ther. 2013; 28:1–8. PMID:
23108779.

8. Hajsadeghi F, Nabavi V, Bhandari A, Choi A, Vincent H, Flores F, et al. Increased epicardial adipose tissue is associated with coronary artery disease and major adverse cardiovascular events. Atherosclerosis. 2014; 237:486–489. PMID:
25463078.

9. Gibbons RJ, Abrams J, Chatterjee K, Daley J, Deedwania PC, Douglas JS, et al. ACC/AHA 2002 guideline update for the management of patients with chronic stable angina--summary article: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on the Management of Patients with Chronic Stable Angina). J Am Coll Cardiol. 2003; 41:159–168. PMID:
12570960.
10. Lee AM, Beaudoin J, Engel LC, Sidhu MS, Abbara S, Brady TJ, et al. Assessment of image quality and radiation dose of prospectively ECG-triggered adaptive dual-source coronary computed tomography angiography (cCTA) with arrhythmia rejection algorithm in systole versus diastole: a retrospective cohort study. Int J Cardiovasc Imaging. 2013; 29:1361–1370. PMID:
23526082.

11. Han Y, Jing J, Tu S, Tian F, Xue H, Chen W, et al. ST elevation acute myocardial infarction accelerates non-culprit coronary lesion atherosclerosis. Int J Cardiovasc Imaging. 2014; 30:253–261. PMID:
24420418.

12. Xia Y, Junjie Y, Ying Z, Bai H, Qi W, Qinhua J, et al. Accuracy of 128-slice dual-source CT using high-pitch spiral mode for the assessment of coronary stents: first in vivo experience. Eur J Radiol. 2013; 82:617–622. PMID:
23265926.

13. Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, Fleg JL, et al. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol. 2014; 64:684–692. PMID:
25125300.
14. Ito H, Motoyama S, Sarai M, Kawai H, Harigaya H, Kan S, et al. Characteristics of plaque progression detected by serial coronary computed tomography angiography. Heart Vessels. 2014; 29:743–749. PMID:
24113717.

15. Hoffmann U, Moselewski F, Nieman K, Jang IK, Ferencik M, Rahman AM, et al. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol. 2006; 47:1655–1662. PMID:
16631006.

16. Gauss S, Achenbach S, Pflederer T, Schuhbäck A, Daniel WG, Marwan M. Assessment of coronary artery remodelling by dual-source CT: a head-to-head comparison with intravascular ultrasound. Heart. 2011; 97:991–997. PMID:
21478387.

17. Kashiwagi M, Tanaka A, Kitabata H, Tsujioka H, Kataiwa H, Komukai K, et al. Feasibility of noninvasive assessment of thin-cap fibroatheroma by multidetector computed tomography. JACC Cardiovasc Imaging. 2009; 2:1412–1419. PMID:
20083077.

18. Ueda H, Harimoto K, Tomoyama S, Tamaru H, Miyawaki M, Mitsusada N, et al. Relation of cardiovascular risk factors and angina status to obstructive coronary artery disease according to categorical coronary artery calcium score. Heart Vessels. 2012; 27:128–134. PMID:
21416117.

19. Bayturan O, Kapadia S, Nicholls SJ, Tuzcu EM, Shao M, Uno K, et al. Clinical predictors of plaque progression despite very low levels of low-density lipoprotein cholesterol. J Am Coll Cardiol. 2010; 55:2736–2742. PMID:
20538166.

20. Rioufol G, Finet G, Ginon I, André-Fouët X, Rossi R, Vialle E, et al. Multiple atherosclerotic plaque rupture in acute coronary syndrome: a three-vessel intravascular ultrasound study. Circulation. 2002; 106:804–808. PMID:
12176951.
21. Asakura M, Ueda Y, Yamaguchi O, Adachi T, Hirayama A, Hori M, et al. Extensive development of vulnerable plaques as a pancoronary process in patients with myocardial infarction: an angioscopic study. J Am Coll Cardiol. 2001; 37:1284–1288. PMID:
11300436.

22. Guazzi MD, Bussotti M, Grancini L, De Cesare N, Guazzi M, Pera IL, et al. Evidence of multifocal activity of coronary disease in patients with acute myocardial infarction. Circulation. 1997; 96:1145–1151. PMID:
9286942.

23. Otsuka K, Fukuda S, Tanaka A, Nakanishi K, Taguchi H, Yoshiyama M, et al. Prognosis of vulnerable plaque on computed tomographic coronary angiography with normal myocardial perfusion image. Eur Heart J Cardiovasc Imaging. 2014; 15:332–340. PMID:
24204033.

24. Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y, et al. Plaque characterization by coronary computed tomography angiography and the likelihood of acute coronary events in mid-term follow-up. J Am Coll Cardiol. 2015; 66:337–346. PMID:
26205589.
25. Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. 2014; 311:271–278. PMID:
24247483.

26. Bourantas CV, Garcia-Garcia HM, Farooq V, Maehara A, Xu K, Généreux P, et al. Clinical and angiographic characteristics of patients likely to have vulnerable plaques: analysis from the PROSPECT study. JACC Cardiovasc Imaging. 2013; 6:1263–1272. PMID:
24269259.
27. Yun KH, Mintz GS, Farhat N, Marso SP, Taglieri N, Verheye S, et al. Relation between angiographic lesion severity, vulnerable plaque morphology and future adverse cardiac events (from the Providing Regional Observations to Study Predictors of Events in the Coronary Tree study). Am J Cardiol. 2012; 110:471–477. PMID:
22579346.

28. Voros S, Rinehart S, Qian Z, Vazquez G, Anderson H, Murrieta L, et al. Prospective validation of standardized, 3-dimensional, quantitative coronary computed tomographic plaque measurements using radiofrequency backscatter intravascular ultrasound as reference standard in intermediate coronary arterial lesions: results from the ATLANTA (assessment of tissue characteristics, lesion morphology, and hemodynamics by angiography with fractional flow reserve, intravascular ultrasound and virtual histology, and noninvasive computed tomography in atherosclerotic plaques) I study. JACC Cardiovasc Interv. 2011; 4:198–208. PMID:
21349459.
29. Marwan M, Taher MA, El Meniawy K, Awadallah H, Pflederer T, Schuhbäck A, et al. In vivo CT detection of lipid-rich coronary artery atherosclerotic plaques using quantitative histogram analysis: a head to head comparison with IVUS. Atherosclerosis. 2011; 215:110–115. PMID:
21227419.

30. Raggi P. Epicardial adipose tissue and progression of coronary artery calcium: cause and effect or simple association? JACC Cardiovasc Imaging. 2014; 7:917–919. PMID:
25212796.
31. Gauss S, Klinghammer L, Steinhoff A, Raaz-Schrauder D, Marwan M, Achenbach S, et al. Association of systemic inflammation with epicardial fat and coronary artery calcification. Inflamm Res. 2015; 64:313–319. PMID:
25763815.

32. Mahabadi AA, Lehmann N, Kälsch H, Robens T, Bauer M, Dykun I, et al. Association of epicardial adipose tissue with progression of coronary artery calcification is more pronounced in the early phase of atherosclerosis: results from the Heinz Nixdorf recall study. JACC Cardiovasc Imaging. 2014; 7:909–916. PMID:
25190138.
33. Park JS, Choi BJ, Choi SY, Yoon MH, Hwang GS, Tahk SJ, et al. Echocardiographically measured epicardial fat predicts restenosis after coronary stenting. Scand Cardiovasc J. 2013; 47:297–302. PMID:
23937273.

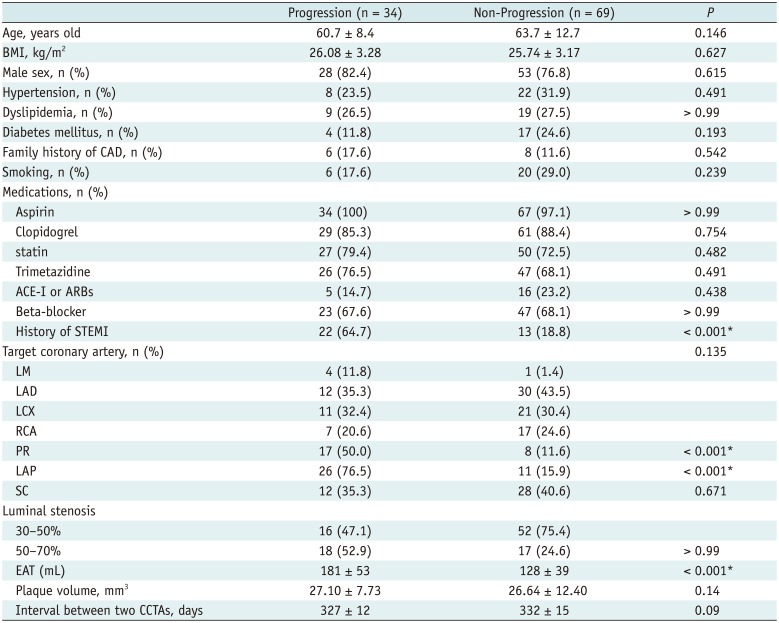
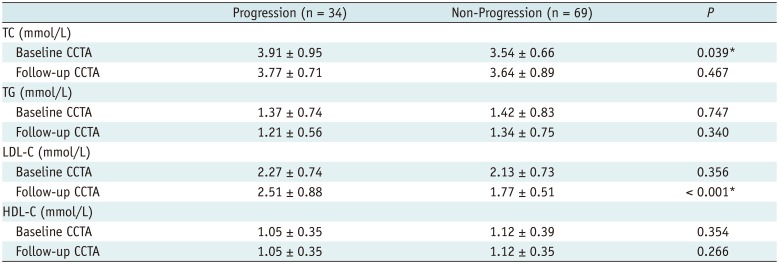
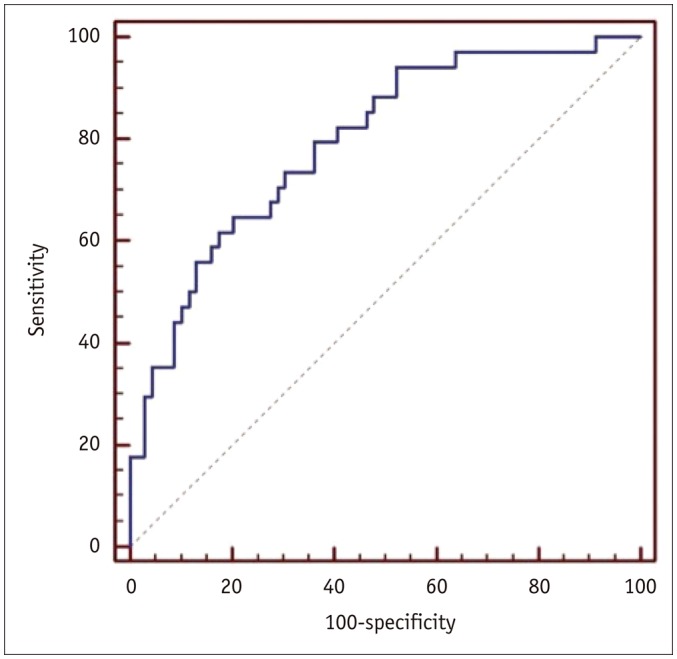
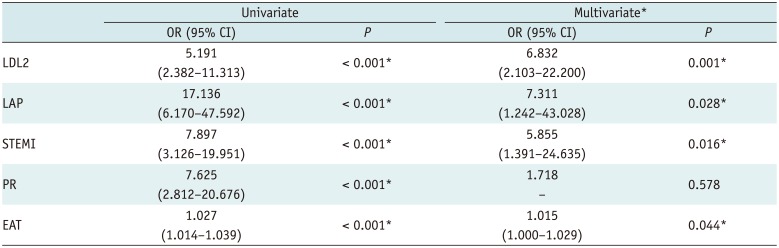




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download