1. Sprague BL, Gangnon RE, Burt V, Trentham-Dietz A, Hampton JM, Wellman RD, et al. Prevalence of mammographically dense breasts in the United States. J Natl Cancer Inst. 2014; 106:dju255. PMID:
25217577.

2. Ko SY, Kim EK, Kim MJ, Moon HJ. Mammographic density estimation with automated volumetric breast density measurement. Korean J Radiol. 2014; 15:313–321. PMID:
24843235.

3. Lee EH, Kim KW, Kim YJ, Shin DR, Park YM, Lim HS, et al. Performance of screening mammography: a report of the alliance for breast cancer screening in Korea. Korean J Radiol. 2016; 17:489–496. PMID:
27390540.

4. Graf O, Berg WA, Sickles EA. Large rodlike calcifications at mammography: analysis of morphologic features. AJR Am J Roentgenol. 2013; 200:299–303. PMID:
23345349.

5. Pollán M, Ascunce N, Ederra M, Murillo A, Erdozáin N, Alés-Martínez J, et al. Mammographic density and risk of breast cancer according to tumor characteristics and mode of detection: a Spanish population-based case-control study. Breast Cancer Res. 2013; 15:R9. PMID:
23360535.

6. Boyd NF, Guo H, Martin LJ, Sun L, Stone J, Fishell E, et al. Mammographic density and the risk and detection of breast cancer. N Engl J Med. 2007; 356:227–236. PMID:
17229950.

7. Mandelson MT, Oestreicher N, Porter PL, White D, Finder CA, Taplin SH, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 2000; 92:1081–1087. PMID:
10880551.

8. Hooley RJ, Greenberg KL, Stackhouse RM, Geisel JL, Butler RS, Philpotts LE. Screening US in patients with mammographically dense breasts: initial experience with Connecticut Public Act 09-41. Radiology. 2012; 265:59–69. PMID:
22723501.

9. Seo M, Cho N, Bae MS, Koo HR, Kim WH, Lee SH, et al. Features of undiagnosed breast cancers at screening breast MR imaging and otential utility of computer-aided evaluation. Korean J Radiol. 2016; 17:59–68. PMID:
26798217.
10. Berg WA. How well does supplemental screening magnetic resonance imaging work in high-risk women? J Clin Oncol. 2014; 32:2193–2196. PMID:
24934782.

11. Moschetta M, Telegrafo M, Rella L, Capolongo A, Stabile Ianora AA, Angelelli G, et al. MR evaluation of breast lesions obtained by diffusion-weighted imaging with background body signal suppression (DWIBS) and correlations with histological findings. Magn Reson Imaging. 2014; 32:605–609. PMID:
24721005.

12. Kul S, Cansu A, Alhan E, Dinc H, Gunes G, Reis A. Contribution of diffusion-weighted imaging to dynamic contrast-enhanced MRI in the characterization of breast tumors. AJR Am J Roentgenol. 2011; 196:210–217. PMID:
21178069.

13. Harvey SC, Di Carlo PA, Lee B, Obadina E, Sippo D, Mullen L. An abbreviated protocol for high-risk screening breast MRI saves time and resources. J Am Coll Radiol. 2016; 13:374–380. PMID:
26521970.

14. Moschetta M, Telegrafo M, Rella L, Stabile Ianora AA, Angelelli G. Abbreviated combined MR protocol: a new faster strategy for characterizing breast lesions. Clin Breast Cancer. 2016; 16:207–211. PMID:
27108218.

15. Grimm LJ, Soo MS, Yoon S, Kim C, Ghate SV, Johnson KS, et al. Abbreviated screening protocol for breast MRI: a feasibility study. Acad Radiol. 2015; 22:1157–1162. PMID:
26152500.
16. Morris EA. Rethinking breast cancer screening: ultra FAST breast magnetic resonance imaging. J Clin Oncol. 2014; 32:2281–2283. PMID:
24958827.

17. Roubidoux MA, Bailey JE, Wray LA, Helvie MA. Invasive cancers detected after breast cancer screening yielded a negative result: relationship of mammographic density to tumor prognostic factors. Radiology. 2004; 230:42–48. PMID:
14695385.

18. Tudorica LA, Oh KY, Roy N, Kettler MD, Chen Y, Hemmingson SL, et al. A feasible high spatiotemporal resolution breast DCE-MRI protocol for clinical settings. Magn Reson Imaging. 2012; 30:1257–1267. PMID:
22770687.

19. Berg WA, Zhang Z, Lehrer D, Jong RA, Pisano ED, Barr RG, et al. Detection of breast cancer with addition of annual screening ultrasound or a single screening MRI to mammography in women with elevated breast cancer risk. JAMA. 2012; 307:1394–1404. PMID:
22474203.

20. Heacock L, Melsaether AN, Heller SL, Gao Y, Pysarenko KM, Babb JS, et al. Evaluation of a known breast cancer using an abbreviated breast MRI protocol: correlation of imaging characteristics and pathology with lesion detection and conspicuity. Eur J Radiol. 2016; 85:815–823. PMID:
26971429.

21. Mango VL, Morris EA, David Dershaw D, Abramson A, Fry C, Moskowitz CS, et al. Abbreviated protocol for breast MRI: are multiple sequences needed for cancer detection? Eur J Radiol. 2015; 84:65–70. PMID:
25454099.

22. Heywang SH, Wolf A, Pruss E, Hilbertz T, Eiermann W, Permanetter W. MR imaging of the breast with Gd-DTPA: use and limitations. Radiology. 1989; 171:95–103. PMID:
2648479.

23. Kuhl CK, Schrading S, Strobel K, Schild HH, Hilgers RD, Bieling HB. Abbreviated breast magnetic resonance imaging (MRI): first postcontrast subtracted images and maximum-intensity projection-a novel approach to breast cancer screening with MRI. J Clin Oncol. 2014; 32:2304–2310. PMID:
24958821.

24. Yoon H, Yoon D, Yun M, Choi JS, Park VY, Kim EK, et al. Metabolomics of breast cancer using high-resolution magic angle spinning magnetic resonance spectroscopy: correlations with 18F-FDG positron emission tomography-computed tomography, dynamic contrast-enhanced and diffusion-weighted imaging MRI. PLoS One. 2016; 11:e0159949. PMID:
27459480.

25. Kızıldağ Yırgın İ, Arslan G, Öztürk E, Yırgın H, Taşdemir N, Gemici AA, et al. Diffusion weighted MR imaging of breast and correlation of prognostic factors in breast cancer. Balkan Med J. 2016; 33:301–307. PMID:
27308074.

26. Sharma U, Sah RG, Agarwal K, Parshad R, Seenu V, Mathur SR, et al. Potential of diffusion-weighted imaging in the characterization of malignant, benign, and healthy breast tissues and molecular subtypes of breast cancer. Front Oncol. 2016; 6:126. PMID:
27242965.

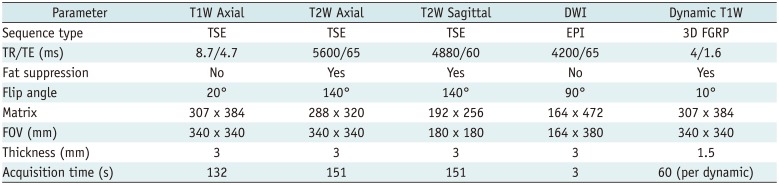
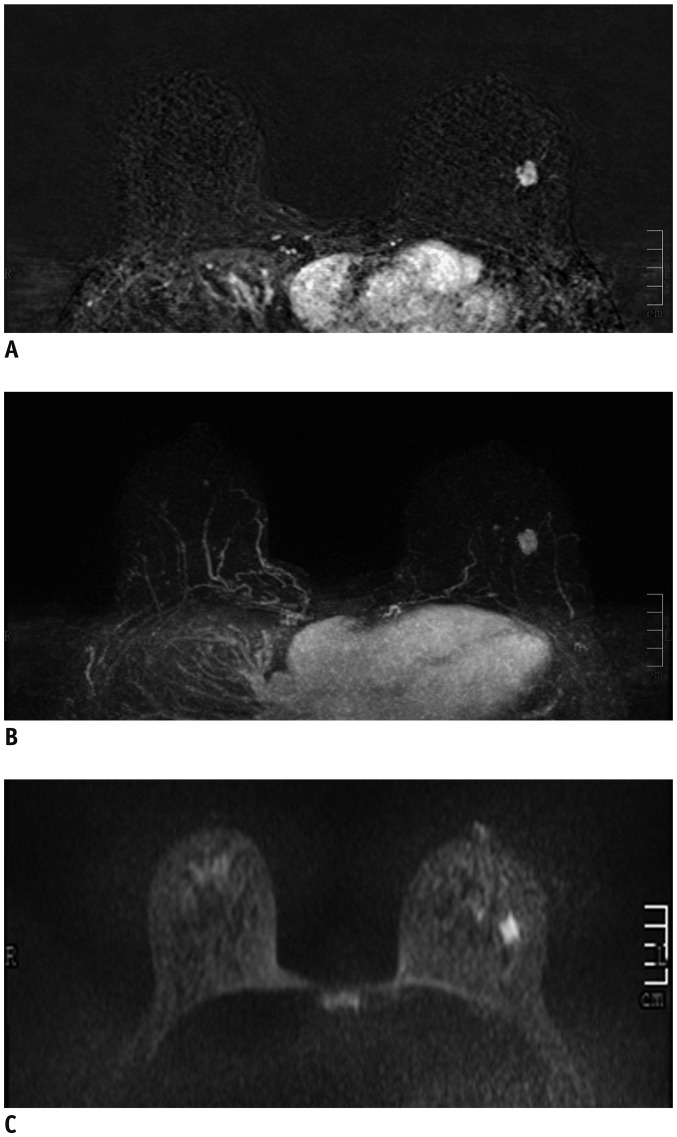
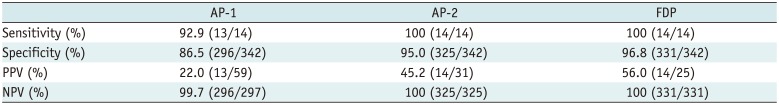




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download