Abstract
Diagnosis of hepatocellular carcinoma (HCC) with gadoxetic acid-enhanced liver magnetic resonance imaging (MRI) poses certain unique challenges beyond the scope of current guidelines. The regional heterogeneity of HCC in demographic characteristics, prevalence, surveillance, and socioeconomic status necessitates different treatment approaches, leading to variations in survival outcomes. Considering the medical practices in Korea, the Korean Society of Abdominal Radiology (KSAR) study group for liver diseases has developed expert consensus recommendations for diagnosis of HCC by gadoxetic acid-enhanced MRI with updated perspectives, using a modified Delphi method. During the 39th Scientific Assembly and Annual Meeting of KSAR (2016), consensus was reached on 12 of 16 statements. These recommendations might serve to ensure a more standardized diagnosis of HCC by gadoxetic acid-enhanced MRI.
Several organizations have published guidelines for imaging diagnosis of hepatocellular carcinoma (HCC), including the American Association for the Study of Liver Diseases (AASLD), European Association for the Study of the Liver–European Organization for Research and Treatment of Cancer (EASL-EORTC), Asian-Pacific Association for the Study of the Liver (APASL), Korean Liver Cancer Study Group-National Cancer Center (KLCSG-NCC), Japan Society of Hepatology (JSH), and American College of Radiology Liver Imaging Reporting and Data Systems (LI-RADS) (12345). Because the diagnostic criteria of HCC are mainly based on its hemodynamic hallmarks, which include hyperenhancement and washout in the hepatic arterial and venous phases, respectively, these guidelines were established based on the assumption of dynamic computed tomography (CT) or magnetic resonance imaging (MRI) with extracellular contrast media (ECCM) being the first-line modality.
Since its initial approval in 2004, gadoxetic acid (gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid or gadoxetate disodium) has been increasingly used as a contrast agent, especially in Asia and Europe. A survey in 2016 revealed 177 of 195 (90.7%) members of the Korean Society of Abdominal Radiology (KSAR) as using gadoxetic acid for MRI in patients with suspected HCC. Several studies have demonstrated the clinical efficacy of gadoxetic acid in early detection of HCC by providing functional information as a hepatocyte-specific contrast agent as well as hemodynamic information. Although ECCMs and gadoxetic acid are both gadolinium-based contrast agents, they differ in terms of pharmacokinetic characteristics, dosage, and mechanism of action.
The regional heterogeneity of HCC in demographic characteristics, prevalence, surveillance, and socioeconomic status necessitates different treatment approaches, leading to variations in survival outcomes, which affects the diagnostic strategy. Korea has a few unique situations which have steered clinicians towards application of gadoxetic acid-enhanced MRI for early diagnosis of HCC; the highest prevalence of HCC, hepatitis B-related chronic liver disease as the most common underlying etiology, variable curative treatment options–especially hepatic resection or radiofrequency ablation–for early HCC, and affordable treatment because of the national health insurance system. However, diagnosis of HCC by gadoxetic acid-enhanced liver MRI poses certain challenges beyond the scope of current guidelines. Therefore, the KSAR organized meetings to reach a consensus on guidelines for diagnosis of HCC by gadoxetic acid-enhanced MRI with updated perspectives and in consideration of current medical practices in Korea.
Five organizing members (M. S. P., J. Y. C., S. Y. K., J. M. L., and Y. K. K.) performed literature review in consensus to collect data regarding diagnosis of HCC by gadoxetic acid-enhanced MRI. The PubMed and MEDLINE databases were searched for relevant original articles, systematic reviews/meta-analyses, and consensus statement/guidelines in English. These data were used to extract relevant topics to be addressed in a questionnaire. Debatable issues that were deemed essential for diagnosis of HCC by gadoxetic acid-enhanced MRI were catalogued. Four panels comprising twenty-one panelists–all members of the KSAR and leading abdominal radiologists with expertise in the field of liver MRI–were each assigned one or two issues of debate. An internist (D. Y. K.), a pathologist (E. S. Y.), and two additional abdominal radiologists (M. J. K. and W. J. L.) were invited as advising members. These panels consolidated relevant evidences regarding their assigned issues and prepared a draft of a specific questionnaire, along with a summary of the clinical and scientific rationale behind their suggestions. The questionnaire was drafted at a face-to-face meeting and refined by online discussion.
The initial 34 questions were presented to members of the KSAR at a one-day symposium (5th Liver Imaging Day; KSAR-Consensus on Diagnosis of HCC with Gadoxetic Acid-enhanced MRI) on April 16, 2016, which involved didactic lectures and a thorough discussion on the issues of debate. A total of 195 board-certified radiologists specializing in abdominal radiology attended this symposium, where the questionnaire was put through first-round voting. The proposed consensus statement was developed using a modified Delphi method based on a six-point scale: strongly agree, agree with minor reservation, agree with major reservation, disagree with minor reservation, disagree with major reservation, and strongly disagree. Consensus was predefined at ≥ 80% of the sum of votes indicating strong agreement or agreement with minor reservation. Of the 34 questionnaire items, 16 achieved consensus. Following the first-round vote, the questionnaire was refined by the panelists by online discussion and put through second-round voting at a half-day satellite conference, attended by 128 board-certified radiologists specializing in abdominal radiology, during the 39th Scientific Assembly and Annual Meeting of the KSAR, May 14, 2016. Finally, 12 of 16 statements reached the 80% consensus threshold (Table 1). All votes were recorded by secret ballot.
Hepatocellular carcinoma can be diagnosed by histopathology or non-invasive imaging (6). In fact, HCC is the only malignancy for which pathologic confirmation is not mandatory for diagnosis. With advances in imaging techniques, reliable assessment can be made based on contrast-enhanced CT or MRI findings.
However, extensive geographical differences in tumor biology and regional tendencies make it challenging to establish universal guidelines for diagnosis of HCC. Moreover, guidelines are influenced by the clinical environment and resources available for treatment. The prevalence of HCC in Korea is higher compared to that in Western countries (7). Patients with positive imaging findings in high-prevalence populations are more likely to have HCC than those in low-prevalence populations. Therefore, differences in disease prevalence might affect the likelihood of diagnosis. Choice of therapy is also influenced by regional and institutional tendencies (8). Because liver transplantation eliminates cancer as well as cirrhotic liver tissue, it is considered as the only curative treatment in many Western countries. While deceased donor-liver transplantation (DDLT) constitutes over 90% of liver transplantation cases in Western countries, most such cases in Korea involve living donor-liver transplantation (910). Since organ shortage remains a major limitation for DDLT, Western guidelines for imaging diagnosis focus on achieving high specificity, comparable to that of histopathologic diagnosis, in order to maximize organ utilization. The United States Organ Procurement and Transplantation Network (OPTN) diagnostic criteria for HCC were specifically designed to improve specificity. In Korea, other treatment approaches, such as surgical resection, radiofrequency ablation, transcatheter arterial therapy, and systemic chemotherapy, are widely used for HCC. Given the vast differences in clinical environment between Western countries and Korea, diagnostic strategies for HCC also differ.
Despite controversies regarding its specificity, accumulating evidence shows that gadoxetic acid-enhanced MRI provides improved sensitivity for detecting HCC (11). In Asian countries where gadoxetic acid-enhanced MRI is widely used, diagnostic criteria for HCC are relaxed to increase sensitivity at the expense of specificity (1213). Corresponding with the emphasis on early detection, Asian guidelines have been developed to address early treatment. Because HCC frequently invades vessels and metastasizes to other parts of the liver and body, aggressive treatment of early-stage HCC improves long-term survival (14). A remaining issue is whether diagnostic sensitivity for HCC can be improved while maintaining acceptable specificity. Further studies are required to refine the current diagnostic criteria for HCC.
In the 2016 KSAR consensus meeting on diagnosis of HCC with gadoxetic acid-enhanced MRI, the consensus level for the following statement was 90%.
Arterial-phase hyperenhancement, a key imaging feature of HCC (215), is observed in 76–82.7% of small HCCs and only 3.2–9.7% of benign nodules (16). It exhibits high positive predictive value (96.5–98.9%) and specificity (90.3–96.8%) but a low negative predictive value (54.6–62.5%) and moderate sensitivity (76–79.8%) (16). Therefore, acquisition of optimal late arterial-phase images is critical for noninvasive imaging diagnosis of HCC. Arterial-phase hyperenhancement on dynamic contrast-enhanced MR images is generally defined as hyperintensity relative to the surrounding liver parenchyma in the arterial phase (1617). However, diagnosis based on this definition often leads to false-positive results in hepatic lesions that already exhibit hyperintensity on unenhanced T1-weighted images because of accumulation of fat, hemosiderin, glycoproteins, or copper (1819). Comparison of unenhanced and arterial-phase images is necessary to avoid this misinterpretation and detect arterial enhancement (20). However, in some instances, it is challenging to detect or determine arterial hyperenhancement by visually comparing two image sets. Additionally, because of the weak enhancement associated with small volumes of contrast media, low gadolinium concentrations (21), and acute transient dyspnea (11), suboptimal arterial enhancement is more frequent with gadoxetic acid-enhanced MRI than with ECCM-MRI. In such cases, subtraction images of unenhanced T1-weighted and arterial-phase images are helpful in detecting arterial-phase hyperenhancement (Fig. 1) (16222324). However, in subtraction imaging, image quality cannot be assured in case of misregistration due to patient-related or technical factors. Nevertheless, subtraction imaging has shown greater diagnostic accuracy than arterial-phase imaging and visual comparison of precontrast and arterial-phase images (16). Therefore, when available, subtraction imaging is recommended for assessment of arterial-phase enhancement.
In the 2016 KSAR consensus meeting, the consensus level for the following statement was 91.2%.
Definition of arterial-phase hyperenhancement should include hyperintensity relative to the surrounding liver parenchyma in the arterial-phase as well greater signal intensity in the arterial phase in comparison with precontrast images (determined by subtraction imaging, when feasible).
Because of its higher relaxivity (25), the standard dosage of gadoxetic acid (0.025 or 0.1 mL/kg) (2126) is half in volume and a quarter in gadolinium concentration of the general dosages of ECCMs. The lower dosage of gadoxetic acid in comparison with those of ECCMs results in a shorter bolus transit time and, thereby, a shorter late arterial-phase window, which necessitates particular attention to the arterial-phase acquisition protocol. Additionally, intravenous gadoxetic acid administration is frequently associated with acute transient dyspnea, which results in severe motion artifacts in 12.9–18% of arterial-phase gadoxetic acid-enhanced MR images (112728). These problems have been partially overcome by modification of contrast injection and imaging protocols, including a slower injection rate (1 mL/s rather than 2 mL/s) (2930), detection of arterial phase by test or fluoroscopic bolus monitoring rather than by fixed-scan delay (3132), and multiple short arterial-phase imaging (123334). However, acquisition of optimal arterial-phase gadoxetic acid-enhanced MR images is still challenging in clinical practice. As a practical solution for this issue, the LI-RADS (v2014) allows substitution of arterial-phase findings on suboptimal gadoxetic acid-enhanced MR images with those on recent CT images (35).
In the 2016 KSAR consensus meeting, the consensus levels for the following two statements were 90% and 88%, respectively.
1. In case of suboptimal gadoxetic acid-enhanced arterial-phase MR images, the findings of recent CT arterial-phase images may be used instead for diagnosis of HCC.
2. Dynamic CT images acquired within a month of gadoxetic acid-enhanced MRI may be considered as appropriate substitutes for suboptimal arterial-phase gadoxetic acid-enhanced MR images.
On ECCM-enhanced CT or MR images, the portal venous (PVP) or delayed phases (DP) are used to determine the presence of washout appearance (36). However, there is controversy regarding the most appropriate phase(s) of gadoxetic acid-enhanced MRI for evaluation of washout appearance (3738). Several latest guidelines that incorporate gadoxetic acid-enhanced MRI in the diagnostic algorithm for HCC permit different phases for identifying the washout pattern. The LI-RADS v2014 permits identification of washout appearance only in the PVP in order to maintain high specificity (515). The KLCSG-NCC guidelines v2014 permit identification of washout appearance in the PVP or transitional phase (TP; usually obtained around 3 minutes after contrast administration) (13). The consensus-based algorithm proposed by the Liver Cancer Study Group of Japan (LCSGJ) v2014 includes gadoxetic acid-enhanced MRI as a first-line imaging modality and permits identification of hypointensity in the TP and hepatobiliary phase (HBP) as an alternative to washout appearance for diagnosis of HCC after exclusion of hemangioma using other sequences of MRI and/or other imaging modalities (39). These discrepancies among different guidelines arise from individual preferences for higher sensitivity or specificity (4041), as mentioned in the “Strategy for the diagnosis of HCC”.
Hypointensity in the HBP is a useful feature for diagnosis of small HCCs, which might have influenced the LCSGJ to include the HBP for evaluation of washout appearance (424344). However, most non-HCC malignancies and non-hepatocyte-containing benign lesions and a proportion of borderline nodules (e.g., dysplastic nodules) show hypointensity in the HBP (364546). Therefore, caution should be given in using hypointensity on the HBP as an alternative to washout appearance, since the former has been reported to result in substantially low specificity (< 50%) for detection of arterial-phase hyper-enhancing nodules of sizes ≥ 1 cm (47).
In comparison to multi-phasic CT, typical washout appearance of HCC on gadoxetic acid-enhanced MRI was less frequent if only PVP was used, while it was more frequent if PVP and/or TP was used (Fig. 2) (4748). However, the main concern regarding inclusion of the TP for determining washout appearance is that, the definition of hypointensity in the TP differs from that in the DP in ECCM-enhanced imaging. Since gadoxetic acid uptake by hepatocytes begins as early as the end of the PVP, hypointensity in the TP might be due to the combined effect of de-enhancement and lack of hepatocyte relative to the surrounding liver parenchyma (3549). This “pseudo-washout” can be observed in high-flow hemangiomas (50) and other non-hepatocyte-containing lesions, which decreases the specificity of diagnosis of HCCs, including hypervascular intrahepatic cholangiocarcinomas (ICC) (475152).
In the 2016 KSAR consensus meeting, in responses to the question “Which phase(s) would be appropriate for determining washout appearance?”, “PVP or TP” gained 85.3% votes, while “PVP only” gained 14.7% votes. The reasons for this choice include: 1) inclusion of the TP would increase the diagnostic sensitivity for HCC, but it would not substantially decrease the positive predictive value,which might be a more suitable parameter in Korea, considering the high prevalence of HCC and low availability of DDLT as a treatment option (4); 2) in most cases, high-flow hemangiomas, which mimic HCCs on dynamic phase images (i.e., arterial hyperenhancement and pseudo-washout in the TP), can be ruled out using other MRI sequences (e.g., T2-weighted [T2W] or diffusion-weighted [DW] imaging with an apparent diffusion coefficient map) (505354); and 3) in patients with hypervascular ICCs, which mimic HCCs on gadoxetic acid-enhanced MR images, prognosis following treatment by the same method as that for equivalent-stage HCC has not been well established (5556). To sum up, the appropriate phase for detection of washout appearance on gadoxetic acid-enhanced MR images should be determined considering the inevitable trade-off between sensitivity and specificity in HCC diagnosis. Additionally, the role of ancillary features and effects on clinical outcomes should also be considered.
The AASLD and EASL-EORTC guidelines do not allow imaging diagnosis of sub-centimeter-sized HCC. Instead of instantaneous diagnosis of sub-centimeter-sized lesions, these guidelines recommend augmented follow-up at short intervals of 3–4 months, as opposed to regular surveillance, typically at 6-month intervals. This recommendation is based on the belief that a majority of nodules of sizes < 1 cm are unlikely to be HCCs (57). Although there are some contrary evidence (585960), over 90% of arterial-enhancing lesions of sizes < 20 mm were found to be non-neoplastic both in patients with and without history of HCC (6162). In addition, the diagnostic performance of imaging studies for smaller HCCs is low. According to a recent meta-analysis, per-lesion sensitivity for diagnosis of HCCs of sizes < 1 cm was significantly lower compared to that for HCCs of sizes ≥ 1 cm (CT, 31% vs. 82%, p < 0.001; MRI, 48% vs. 88%, p = 0.02) (4263). Even when sub-centimeter-sized lesions are diagnosed on CT or MR images, it would be tricky to co-localize them for intervention or surgery (6465).
In comparison with other imaging modalities, gadoxetic acid-enhanced MRI provides a greater opportunity for detection of small or early HCCs (13), which is supported by evidence that gadoxetic acid-enhanced MRI outperforms CT and ECCM-MRI in diagnosis of lesions of sizes < 1–2 cm (66676869). Moreover, a significant proportion of sub-centimeter-sized lesions detected by gadoxetic acid-enhanced MRI are likely to be or turn into HCC within a short time period (7071). In accordance with these new findings, several recent guidelines, including those of the KLCSG-NCC, JSH, APASL, and LI-RADS v2014, allow imaging diagnosis of sub-centimeter-sized HCC. Given that the mainstay treatment for HCC in Korea is locoregional treatment rather than liver transplantation, detection of smaller lesions susceptible to locoregional v2014, allow imaging diagnosis appears meaningful (72737475). Additionally, new diagnostic techniques, such as fusion of real-time ultrasonography (US) images with CT/MR or contrast-enhanced US images, have made it possible to accurately localize small lesions for local treatment (76), thus bridging the distance between gadoxetic acid-enhanced liver MRI and optimal treatment.
Despite the greater efficacy of gadoxetic acid-enhanced MRI in comparison with those of conventional imaging modalities for diagnosis of sub-centimeter-sized HCC, the diagnostic performance of gadoxetic acid-enhanced liver MRI alone is still unsatisfactory (6377). Recent studies have reported that inclusion of ancillary imaging features, including moderate hyperintensity on T2W imaging (T2WI), restricted diffusion, and hypointensity in the HBP, along with the typical vascular profile changes of HCC improves the diagnostic performance of gadoxetic acid-enhanced liver MRI in small HCCs (Fig. 3) (637177787980). Therefore, typical vascular profile changes should not be relied on as the sole criteria for diagnosis of sub-centimeter-sized HCC.
Besides, the clinical benefits of treatment of sub-centimeter-sized lesions are yet to be proven. Although lesion size of 2 cm has been suggested as being indicative of aggressiveness and invasiveness in HCC (748182), it is not clear whether the same may be extrapolated to sub-centimeter-sized lesions. Therefore, caution should be exercised in diagnosis of sub-centimeter-sized HCC, because the additional cost and possibility of false-positive diagnosis could offset its potential clinical benefits.
In the 2016 KSAR consensus meeting, the consensus levels for the following two statements were 86% and 98%, respectively.
1. Sub-centimeter-sized HCC may be diagnosed by gadoxetic acid-enhanced liver MRI by applying additional refined diagnostic criteria in addition to the typical vascular profile changes.
2. Additional diagnostic criteria include ancillary MRI findings such as moderate hyperintensity on T2WI, restricted diffusion, and hypointensity in the HBP.
While some guidelines, including those of the EASL-EORTC and LI-RADS v2014, have provided different diagnostic criteria for nodules of sizes ranging from 1 to 2 cm and for those with sizes > 2 cm, other guidelines, including those of the AASLD, KLCSG-NCC, JSH, and APASL, have not. Because of the concern that nodules of sizes < 2 cm are more likely to be benign lesions than HCC (6162), guidelines such as those of the EASL-EORTC and LI-RADS v2014 have implemented stricter criteria for such lesions in order to maintain high diagnostic specificity. The previous version of the AASLD guidelines required coincidental positivity on two imaging modalities for lesions < 2 cm in size. However, since sequential use of a single imaging modality exhibits similar specificity as simultaneous imaging, with substantially reduced resource expenditure, this policy was discarded in the latest version of the AASLD guidelines (8384). Furthermore, the findings of recent meta-analyses revealed gadoxetic acid-enhanced MRI as exhibiting excellent diagnostic performance for lesions < 2 cm in size (sensitivity, 79–95%; specificity, 89–92%) (6985). Thus, in the era of gadoxetic acid-enhanced MRI, the necessity of the 2-cm cut-off appears to be diminishing.
In the 2016 KSAR consensus meeting, the following statement received 86% votes.
Non-hypervascular hypointense nodules in the HPB is an important issue with gadoxetic acid-enhanced MRI. Several studies have suggested that expression of organic anion transporting polypeptide 1B1/3 (OATP 1B1/3) decreases with tumor progression, which can be assessed using hepatocyte-specific MR contrast agents (364886). Given that OATP 1B1/3 expression decreases prior to angiogenesis, hypointense HBP nodules appear prior to HCCs with typical hemodynamic hallmarks, which proves the feasibility of gadoxetic acid-enhanced MRI in detecting early HCC (41488788). Although the clinical impact of these non-hypervascular HBP hypointense nodules in cirrhotic patients is not yet clearly defined, several papers reported that a substantial proportion of nonhypervascular HBP hypointense nodules (≥ 1 cm) were pathologically diagnosed as early HCCs followed by high-grade dysplastic nodules (HGDNs) (8990), up to 30% of nodules were found to have transformed to typical hypervascular HCCs on follow-up imaging within 3 years (899192939495); additionally, patients with non-hypervascular HBP hypointense nodules exhibited shorter recurrence-free survival after radiofrequency ablation and lower overall survival after liver resection than those without (9697).
However, most guidelines, except JSH or APASL, do not recommend noninvasive imaging diagnosis of non-hypervascular HBP hypointense nodules (23) because of the considerable overlap in features between early HCCs and HGDNs as well as the very low specificity (4148899899100101). Several ancillary features, including restricted diffusion, mild to moderate T2 hyperintensity, diameter > 1.5 cm, and presence of fat, are useful for cross-sectional or longitudinal characterization of non-hypervascular HBP hypointense nodules (899092102103104105106107).
At present, management for patients with non-hypervascular HBP hypointense nodules is controversial and includes several options, such as biopsy, intense follow-up, and additional studies such as contrast-enhanced US (199). Although non-hypervascular HBP hypointense nodules have a probability of transforming into malignant or premalignant nodules, they do not have to be treated as urgently as hypervascular HCCs (99108). A recent study demonstrated simultaneous resection of concomitant non-hypervascular HBP nodules with typical hypervascular HCCs could provide significant benefit for recurrence-free survival (109). However, another study reported only marginal survival benefit from resection of early HCCs (110). Therefore, further studies on management of non-hypervascular HBP hypointensenodule are required.
In the 2016 KSAR consensus meeting, the following statements received 86% and 83% votes, respectively.
1. Non-hypervascular HBP hypointense nodules with mild to moderate T2 hyperintensity and/or restricted diffusion should be considered potentially malignant.
2. Strategies for diagnosis and management of non-hypervascular HBP hypointense nodules should vary according to previous or concomitant HCC.
Capsular appearance is defined by the LI-RADS guidelines v2014 as a “peripheral rim of smooth hyper-enhancement in the PVP or DP that unequivocally is thicker or more conspicuous than the rims surrounding background nodule” (5). Capsular appearance is more difficult to recognize with gadoxetic acid-enhanced MRI than with ECCM-enhanced MRI, because early gadoxetic acid uptake by hepatocytes leads to early appearance of strong liver parenchyma enhancement in the PVP and/or TP, which, in turn, obscures any capsular rim enhancement (35). Because of this difference in pharmacodynamics characteristics, capsular appearance should be defined differently on gadoxetic acid-enhanced and ECCM-enhanced MR images. In a recent pathologic correlation study, presence of a smooth dark rim in the HBP was found to exhibit greater correlation with presence of histologic capsule than with conventional capsular appearance on the PVP or TP (76.1% vs. 59.4%; p < 0.001) (Fig. 4) (111). This capsular appearance in the HBP corresponds to HBP hypointense rim, a new, but not major, ancillary feature favoring malignancy described in the LI-RADS v2014 lexicon (35). Capsular appearance has been presented as one of the major features of HCC in the LI-RADS and OPTN guidelines. However, most other current imaging-based diagnostic guidelines do not include capsular appearance as a major feature for HCC diagnosis mainly because of its lack of additional diagnostic value (18). Additionally, interobserver agreement on capsular appearance has been reported as being merely moderate (19).
In the 2016 KSAR consensus meeting, the statement that “capsular appearance is better considered an ancillary feature than a major feature for diagnosis of HCC” received 79.7% votes, while the following statement received 85.3% votes.
In view of the limitations of the current HCC diagnostic criteria, which rely on enhancement patterns, addition of a variety of ancillary features would be reasonable method for improving diagnostic accuracy for HCC. Because HCC on gadoxetic acid-enhanced MR images might exhibit different enhancement patterns from that on ECCM-enhanced MR images, other features apart from enhancement patterns could be important for diagnosis of HCC.
Although they are not specific for HCC, restricted diffusion and mild to moderate T2 hyperintensity are important ancillary features for differentiating between malignant and benign lesions (Fig. 5). However, the sensitivities of these features are not high because several well-differentiated HCCs and some small moderately-differentiated HCCs exhibit iso or hypointensity on T2W and DW images (36). Moreover, DW imaging for diagnosis of HCC presents additional issues. First, background fibrotic parenchyma frequently exhibit lower diffusivity than normal liver, thereby, reducing the lesion–liver contrast on DW images. Second, DW images are prone to spatial distortion and motion artifacts, which make reliable evaluation of hepatic lesions challenging, especially in the left lateral hepatic section, where artifacts due to cardiac motion are inevitable. Third, small HCCs and hemangiomas might exhibit overlapping DW signal intensities (112). For these reasons, the KSAR members could not reach consensus (74%) on the additional role of restricted diffusion and mild-moderate T2 hyperintensity as ancillary features for differentiating HCCs from premalignant nodules under typical circumstances; however, this criterion achieved consensus under special preconditions, including sub-centimeter-sized nodules with typical vascular profile changes (98%) and non-hypervascular HBP hypointense nodules (86.4%) in high-risk populations.
The LI-RADS guidelines are the only ones to incorporate a variety of ancillary features that might favor HCC or benign nodules by a more detailed evaluation of imaging findings (9). The LI-RADS v2014 ancillary features that might favor malignancy include HBP and TP hypointensity, mild to moderate T2 hyperintensity, restricted diffusion, distinctive rim, corona enhancement, mosaic and nodule-in-nodule architecture, intra-lesional fat, lesional iron and fat sparing, blood products, and diameter increase less than the threshold (5). Of these features, distinctive rim, coronal enhancement, mosaic and nodule-in-nodule architecture, and intra-lesional fat are considered as specifically favoring HCC over malignancies (5). These ancillary features in the LI-RADS guidelines are intended to modify the likelihood of diagnosis of HCC but not to upgrade HCC category to LR5 (definitely HCC) without any weighted value on individual features. However, since these ancillary features vary in frequency and importance and are challenging to detect by gadoxetic acid-enhanced MRI, the KSAR members reached an 86% consensus for the following statement.
Despite its better diagnostic performance for HCC in comparison with other imaging modalities, most current guidelines have neither accepted gadoxetic acid-enhanced MRI as a mainstream diagnostic algorithm nor defined standard criteria for diagnosis of HCC by this method. The 2016 KSAR meeting reached consensus on several issues of debate from the radiologists' point of view, based on routine clinical practices. Although several challenges remain in terms of optimization and standardization, these consensus recommendations might serve as useful tools to ensure more standardized diagnosis of HCC by gadoxetic acid-enhanced MRI.
Appendix
Appendix
Author List (in Alphabetical Order) and Affiliations
References
1. Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y, et al. JSH consensus-based clinical practice guidelines for the management of hepatocellular carcinoma: 2014 update by the Liver Cancer Study Group of Japan. Liver Cancer. 2014; 3:458–468. PMID: 26280007.

2. European Association for the Study of the Liver. European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012; 56:908–943. PMID: 22424438.
3. Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011; 53:1020–1022. PMID: 21374666.

4. Korean Liver Cancer Study Group (KLCSG). National Cancer Center, Korea (NCC). 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015; 16:465–522. PMID: 25995680.
5. American College of Radiology. Liver Imaging Reporting and Data System (LI-RADS) version 2014. Accessed December 1, 2016. Available at: https://www.acr.org/Quality-Safety/Resources/LIRADS.
6. Sherman M. The radiological diagnosis of hepatocellular carcinoma. Am J Gastroenterol. 2010; 105:610–612. PMID: 20203642.

8. Graf D, Vallböhmer D, Knoefel WT, Kröpil P, Antoch G, Sagir A, et al. Multimodal treatment of hepatocellular carcinoma. Eur J Intern Med. 2014; 25:430–437. PMID: 24666568.

9. Shukla A, Vadeyar H, Rela M, Shah S. Liver transplantation: East versus West. J Clin Exp Hepatol. 2013; 3:243–253. PMID: 25755506.

10. de Villa V, Lo CM. Liver transplantation for hepatocellular carcinoma in Asia. Oncologist. 2007; 12:1321–1331. PMID: 18055852.
11. Davenport MS, Viglianti BL, Al-Hawary MM, Caoili EM, Kaza RK, Liu PS, et al. Comparison of acute transient dyspnea after intravenous administration of gadoxetate disodium and gadobenate dimeglumine: effect on arterial phase image quality. Radiology. 2013; 266:452–461. PMID: 23192781.

12. Kagawa Y, Okada M, Kumano S, Katsube T, Imaoka I, Tanigawa N, et al. Optimal scanning protocol of arterial dominant phase for hypervascular hepatocellular carcinoma with gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced MR. J Magn Reson Imaging. 2011; 33:864–872. PMID: 21448951.

13. Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part I. Development, growth, and spread: key pathologic and imaging aspects. Radiology. 2014; 272:635–654. PMID: 25153274.

14. Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005; 40:225–235. PMID: 15830281.

15. Mitchell DG, Bruix J, Sherman M, Sirlin CB. LI-RADS (Liver Imaging Reporting and Data System): summary, discussion, and consensus of the LI-RADS Management Working Group and future directions. Hepatology. 2015; 61:1056–1065. PMID: 25041904.

16. An C, Park MS, Kim D, Kim YE, Chung WS, Rhee H, et al. Added value of subtraction imaging in detecting arterial enhancement in small (< 3 cm) hepatic nodules on dynamic contrast-enhanced MRI in patients at high risk of hepatocellular carcinoma. Eur Radiol. 2013; 23:924–930. PMID: 23138382.
17. Nino-Murcia M, Olcott EW, Jeffrey RB Jr, Lamm RL, Beaulieu CF, Jain KA. Focal liver lesions: pattern-based classification scheme for enhancement at arterial phase CT. Radiology. 2000; 215:746–751. PMID: 10831693.

18. Ebara M, Fukuda H, Kojima Y, Morimoto N, Yoshikawa M, Sugiura N, et al. Small hepatocellular carcinoma: relationship of signal intensity to histopathologic findings and metal content of the tumor and surrounding hepatic parenchyma. Radiology. 1999; 210:81–88. PMID: 9885591.

19. Kadoya M, Matsui O, Takashima T, Nonomura A. Hepatocellular carcinoma: correlation of MR imaging and histopathologic findings. Radiology. 1992; 183:819–825. PMID: 1316622.

20. Lutz AM, Willmann JK, Goepfert K, Marincek B, Weishaupt D. Hepatocellular carcinoma in cirrhosis: enhancement patterns at dynamic gadolinium- and superparamagnetic iron oxide-enhanced T1-weighted MR imaging. Radiology. 2005; 237:520–528. PMID: 16192317.

21. Reimer P, Rummeny EJ, Shamsi K, Balzer T, Daldrup HE, Tombach B, et al. Phase II clinical evaluation of Gd-EOB-DTPA: dose, safety aspects, and pulse sequence. Radiology. 1996; 199:177–183. PMID: 8633143.

22. Seçil M, Obuz F, Altay C, Gencel O, Iğci E, Sağol O, et al. The role of dynamic subtraction MRI in detection of hepatocellular carcinoma. Diagn Interv Radiol. 2008; 14:200–204. PMID: 19061165.
23. Winters SD, Jackson S, Armstrong GA, Birchall IW, Lee KH, Low G. Value of subtraction MRI in assessing treatment response following image-guided loco-regional therapies for hepatocellular carcinoma. Clin Radiol. 2012; 67:649–655. PMID: 22300821.

24. Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of hepatocellular carcinoma in the Asia-Pacific region. Gut Liver. 2016; 10:332–339. PMID: 27114433.

25. Rohrer M, Bauer H, Mintorovitch J, Requardt M, Weinmann HJ. Comparison of magnetic properties of MRI contrast media solutions at different magnetic field strengths. Invest Radiol. 2005; 40:715–724. PMID: 16230904.

26. Hamm B, Staks T, Mühler A, Bollow M, Taupitz M, Frenzel T, et al. Phase I clinical evaluation of Gd-EOB-DTPA as a hepatobiliary MR contrast agent: safety, pharmacokinetics, and MR imaging. Radiology. 1995; 195:785–792. PMID: 7754011.

27. Davenport MS, Caoili EM, Kaza RK, Hussain HK. Matched within-patient cohort study of transient arterial phase respiratory motion-related artifact in MR imaging of the liver: gadoxetate disodium versus gadobenate dimeglumine. Radiology. 2014; 272:123–131. PMID: 24617733.

28. Kim SY, Park SH, Wu EH, Wang ZJ, Hope TA, Chang WC, et al. Transient respiratory motion artifact during arterial phase MRI with gadoxetate disodium: risk factor analyses. AJR Am J Roentgenol. 2015; 204:1220–1227. PMID: 26001231.

29. Tamada T, Ito K, Yoshida K, Kanki A, Higaki A, Tanimoto D, et al. Comparison of three different injection methods for arterial phase of Gd-EOB-DTPA enhanced MR imaging of the liver. Eur J Radiol. 2011; 80:e284–e288. PMID: 21296514.

30. Zech CJ, Vos B, Nordell A, Urich M, Blomqvist L, Breuer J, et al. Vascular enhancement in early dynamic liver MR imaging in an animal model: comparison of two injection regimen and two different doses Gd-EOB-DTPA (gadoxetic acid) with standard Gd-DTPA. Invest Radiol. 2009; 44:305–310. PMID: 19462484.

31. Haradome H, Grazioli L, Tsunoo M, Tinti R, Frittoli B, Gambarini S, et al. Can MR fluoroscopic triggering technique and slow rate injection provide appropriate arterial phase images with reducing artifacts on gadoxetic acid-DTPA (Gd-EOB-DTPA)-enhanced hepatic MR imaging? J Magn Reson Imaging. 2010; 32:334–340. PMID: 20677259.

32. Nakamura S, Nakaura T, Kidoh M, Utsunomiya D, Doi Y, Harada K, et al. Timing of the hepatic arterial phase at Gd-EOB-DTPA-enhanced hepatic dynamic MRI: comparison of the test-injection and the fixed-time delay method. J Magn Reson Imaging. 2013; 38:548–554. PMID: 23744782.

33. Fujinaga Y, Ohya A, Tokoro H, Yamada A, Ueda K, Ueda H, et al. Radial volumetric imaging breath-hold examination (VIBE) with k-space weighted image contrast (KWIC) for dynamic gadoxetic acid (Gd-EOB-DTPA)-enhanced MRI of the liver: advantages over Cartesian VIBE in the arterial phase. Eur Radiol. 2014; 24:1290–1299. PMID: 24633374.

34. Pietryga JA, Burke LM, Marin D, Jaffe TA, Bashir MR. Respiratory motion artifact affecting hepatic arterial phase imaging with gadoxetate disodium: examination recovery with a multiple arterial phase acquisition. Radiology. 2014; 271:426–434. PMID: 24475864.

35. Hope TA, Fowler KJ, Sirlin CB, Costa EA, Yee J, Yeh BM, et al. Hepatobiliary agents and their role in LI-RADS. Abdom Imaging. 2015; 40:613–625. PMID: 25287679.

36. Choi JY, Lee JM, Sirlin CB. CT and MR imaging diagnosis and staging of hepatocellular carcinoma: part II. Extracellular agents, hepatobiliary agents, and ancillary imaging features. Radiology. 2014; 273:30–35. PMID: 25247563.

37. Choi SH, Byun JH, Lim YS, Yu E, Lee SJ, Kim SY, et al. Diagnostic criteria for hepatocellular carcinoma ≤ 3 cm with hepatocyte-specific contrast-enhanced magnetic resonance imaging. J Hepatol. 2016; 64:1099–1107. PMID: 26820629.
38. Joo I, Lee JM, Lee DH, Jeon JH, Han JK, Choi BI. Noninvasive diagnosis of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: can hypointensity on the hepatobiliary phase be used as an alternative to washout? Eur Radiol. 2015; 25:2859–2868. PMID: 25773941.

39. Kudo M, Matsui O, Izumi N, Iijima H, Kadoya M, Imai Y. Liver Cancer Study Group of Japan. Surveillance and diagnostic algorithm for hepatocellular carcinoma proposed by the Liver Cancer Study Group of Japan: 2014 update. Oncology. 2014; 87(Suppl 1):7–21.

40. Joo I, Lee JM. Recent advances in the imaging diagnosis of hepatocellular carcinoma: value of gadoxetic acid-enhanced MRI. Liver Cancer. 2016; 5:67–87. PMID: 26989660.

41. Yoon JH, Park JW, Lee JM. Noninvasive diagnosis of hepatocellular carcinoma: elaboration on Korean Liver Cancer Study Group-National Cancer Center Korea practice guidelines compared with other guidelines and remaining issues. Korean J Radiol. 2016; 17:7–24. PMID: 26798212.

42. Lee YJ, Lee JM, Lee JS, Lee HY, Park BH, Kim YH, et al. Hepatocellular carcinoma: diagnostic performance of multidetector CT and MR imaging-a systematic review and meta-analysis. Radiology. 2015; 275:97–109. PMID: 25559230.

43. Haradome H, Grazioli L, Tinti R, Morone M, Motosugi U, Sano K, et al. Additional value of gadoxetic acid-DTPA-enhanced hepatobiliary phase MR imaging in the diagnosis of early-stage hepatocellular carcinoma: comparison with dynamic triple-phase multidetector CT imaging. J Magn Reson Imaging. 2011; 34:69–78. PMID: 21598343.

44. Lee DH, Lee JM, Baek JH, Shin CI, Han JK, Choi BI. Diagnostic performance of gadoxetic acid-enhanced liver MR imaging in the detection of HCCs and allocation of transplant recipients on the basis of the Milan criteria and UNOS guidelines: correlation with histopathologic findings. Radiology. 2015; 274:149–160. PMID: 25203131.

45. Channual S, Tan N, Siripongsakun S, Lassman C, Lu DS, Raman SS. Gadoxetate disodium-enhanced MRI to differentiate dysplastic nodules and grade of hepatocellular carcinoma: correlation with histopathology. AJR Am J Roentgenol. 2015; 205:546–553. PMID: 26295640.

46. Jeong WK, Kim YK, Song KD, Choi D, Lim HK. The MR imaging diagnosis of liver diseases using gadoxetic acid: emphasis on hepatobiliary phase. Clin Mol Hepatol. 2013; 19:360–366. PMID: 24459639.

47. Joo I, Lee JM, Lee DH, Ahn SJ, Lee ES, Han JK. Liver imaging reporting and data system v2014 categorization of hepatocellular carcinoma on gadoxetic acid-enhanced MRI: comparison with multiphasic multidetector computed tomography. J Magn Reson Imaging. 2017; 45:731–740. PMID: 27474328.

48. Park VY, Choi JY, Chung YE, Kim H, Park MS, Lim JS, et al. Dynamic enhancement pattern of HCC smaller than 3 cm in diameter on gadoxetic acid-enhanced MRI: comparison with multiphasic MDCT. Liver Int. 2014; 34:1593–1602. PMID: 24673802.
49. Shah A, Tang A, Santillan C, Sirlin C. Cirrhotic liver: what's that nodule? The LI-RADS approach. J Magn Reson Imaging. 2016; 43:281–294. PMID: 25996905.

50. Doo KW, Lee CH, Choi JW, Lee J, Kim KA, Park CM. “Pseudo washout” sign in high-flow hepatic hemangioma on gadoxetic acid contrast-enhanced MRI mimicking hypervascular tumor. AJR Am J Roentgenol. 2009; 193:W490–W496. PMID: 19933623.

51. Péporté AR, Sommer WH, Nikolaou K, Reiser MF, Zech CJ. Imaging features of intrahepatic cholangiocarcinoma in Gd-EOB-DTPA-enhanced MRI. Eur J Radiol. 2013; 82:e101–e106. PMID: 23159401.

52. Kang Y, Lee JM, Kim SH, Han JK, Choi BI. Intrahepatic mass-forming cholangiocarcinoma: enhancement patterns on gadoxetic acid-enhanced MR images. Radiology. 2012; 264:751–760. PMID: 22798225.

53. Nam SJ, Yu JS, Cho ES, Kim JH, Chung JJ. High-flow haemangiomas versus hypervascular hepatocellular carcinoma showing “pseudo-washout” on gadoxetic acid-enhanced hepatic MRI: value of diffusion-weighted imaging in the differential diagnosis of small lesions. Clin Radiol. 2017; 72:247–254. PMID: 27789027.

54. Outwater EK, Ito K, Siegelman E, Martin CE, Bhatia M, Mitchell DG. Rapidly enhancing hepatic hemangiomas at MRI: distinction from malignancies with T2-weighted images. J Magn Reson Imaging. 1997; 7:1033–1039. PMID: 9400846.

55. Takahashi K, Obeid J, Burmeister CS, Bruno DA, Kazimi MM, Yoshida A, et al. Intrahepatic cholangiocarcinoma in the liver explant after liver transplantation: histological differentiation and prognosis. Ann Transplant. 2016; 21:208–215. PMID: 27068242.

56. Kim JH, Won HJ, Shin YM, Kim KA, Kim PN. Radiofrequency ablation for the treatment of primary intrahepatic cholangiocarcinoma. AJR Am J Roentgenol. 2011; 196:W205–W209. PMID: 21257864.

57. Roskams T. Anatomic pathology of hepatocellular carcinoma: impact on prognosis and response to therapy. Clin Liver Dis. 2011; 15:245–259. vii–x. PMID: 21689611.

58. Park MJ, Kim YS, Lee WJ, Lim HK, Rhim H, Lee J. Outcomes of follow-up CT for small (5-10-mm) arterially enhancing nodules in the liver and risk factors for developing hepatocellular carcinoma in a surveillance population. Eur Radiol. 2010; 20:2397–2404. PMID: 20559837.

59. Forner A, Vilana R, Ayuso C, Bianchi L, Solé M, Ayuso JR, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008; 47:97–104. PMID: 18069697.

60. Hwang SH, Yu JS, Kim KW, Kim JH, Chung JJ. Small hypervascular enhancing lesions on arterial phase images of multiphase dynamic computed tomography in cirrhotic liver: fate and implications. J Comput Assist Tomogr. 2008; 32:39–45. PMID: 18303286.
61. Holland AE, Hecht EM, Hahn WY, Kim DC, Babb JS, Lee VS, et al. Importance of small (< or = 20-mm) enhancing lesions seen only during the hepatic arterial phase at MR imaging of the cirrhotic liver: evaluation and comparison with whole explanted liver. Radiology. 2005; 237:938–944. PMID: 16306035.
62. Byrnes V, Shi H, Kiryu S, Rofsky NM, Afdhal NH. The clinical outcome of small (< 20 mm) arterially enhancing nodules on MRI in the cirrhotic liver. Am J Gastroenterol. 2007; 102:1654–1659. PMID: 17521396.
63. Yu MH, Kim JH, Yoon JH, Kim HC, Chung JW, Han JK, et al. Small (≤ 1-cm) hepatocellular carcinoma: diagnostic performance and imaging features at gadoxetic acid-enhanced MR imaging. Radiology. 2014; 271:748–760. PMID: 24588677.
64. Lee MW, Kim YJ, Park HS, Yu NC, Jung SI, Ko SY, et al. Targeted sonography for small hepatocellular carcinoma discovered by CT or MRI: factors affecting sonographic detection. AJR Am J Roentgenol. 2010; 194:W396–W400. PMID: 20410384.

65. Kim PN, Choi D, Rhim H, Rha SE, Hong HP, Lee J, et al. Planning ultrasound for percutaneous radiofrequency ablation to treat small (≤ 3 cm) hepatocellular carcinomas detected on computed tomography or magnetic resonance imaging: a multicenter prospective study to assess factors affecting ultrasound visibility. J Vasc Interv Radiol. 2012; 23:627–634. PMID: 22387030.

66. Kim SH, Kim SH, Lee J, Kim MJ, Jeon YH, Park Y, et al. Gadoxetic acid-enhanced MRI versus triple-phase MDCT for the preoperative detection of hepatocellular carcinoma. AJR Am J Roentgenol. 2009; 192:1675–1681. PMID: 19457834.

67. Sano K, Ichikawa T, Motosugi U, Sou H, Muhi AM, Matsuda M, et al. Imaging study of early hepatocellular carcinoma: usefulness of gadoxetic acid-enhanced MR imaging. Radiology. 2011; 261:834–844. PMID: 21998047.

68. Chen L, Zhang L, Bao J, Zhang J, Li C, Xia Y, et al. Comparison of MRI with liver-specific contrast agents and multidetector row CT for the detection of hepatocellular carcinoma: a meta-analysis of 15 direct comparative studies. Gut. 2013; 62:1520–1521. PMID: 23929696.

69. Kierans AS, Kang SK, Rosenkrantz AB. The diagnostic performance of dynamic contrast-enhanced MR imaging for detection of small hepatocellular carcinoma measuring up to 2 cm: a meta-analysis. Radiology. 2016; 278:82–94. PMID: 26098460.

70. Song KD, Kim SH, Lim HK, Jung SH, Sohn I, Kim HS. Subcentimeter hypervascular nodule with typical imaging findings of hepatocellular carcinoma in patients with history of hepatocellular carcinoma: natural course on serial gadoxetic acid-enhanced MRI and diffusion-weighted imaging. Eur Radiol. 2015; 25:2789–2796. PMID: 25735515.

71. Jang KM, Kim SH, Kim YK, Choi D. Imaging features of subcentimeter hypointense nodules on gadoxetic acid-enhanced hepatobiliary phase MR imaging that progress to hypervascular hepatocellular carcinoma in patients with chronic liver disease. Acta Radiol. 2015; 56:526–535. PMID: 24838304.

72. Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, et al. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: is resection still the treatment of choice? Hepatology. 2008; 47:82–89. PMID: 18008357.

73. Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, et al. The Liver Cancer Study Group of Japan. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. Hepatology. 2000; 32:1224–1229. PMID: 11093728.

74. Takayama T, Makuuchi M, Hirohashi S, Sakamoto M, Yamamoto J, Shimada K, et al. Early hepatocellular carcinoma as an entity with a high rate of surgical cure. Hepatology. 1998; 28:1241–1246. PMID: 9794907.

75. Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, et al. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005; 234:954–960. PMID: 15681691.

76. Kang TW, Rhim H. Recent advances in tumor ablation for hepatocellular carcinoma. Liver Cancer. 2015; 4:176–187. PMID: 26674766.

77. Park MJ, Kim YK, Lee MW, Lee WJ, Kim YS, Kim SH, et al. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology. 2012; 264:761–770. PMID: 22843769.

78. Lee MH, Kim SH, Park MJ, Park CK, Rhim H. Gadoxetic acid-enhanced hepatobiliary phase MRI and high-b-value diffusion-weighted imaging to distinguish well-differentiated hepatocellular carcinomas from benign nodules in patients with chronic liver disease. AJR Am J Roentgenol. 2011; 197:W868–W875. PMID: 22021534.

79. Kim JE, Kim SH, Lee SJ, Rhim H. Hypervascular hepatocellular carcinoma 1 cm or smaller in patients with chronic liver disease: characterization with gadoxetic acid-enhanced MRI that includes diffusion-weighted imaging. AJR Am J Roentgenol. 2011; 196:W758–W765. PMID: 21606265.

80. Park MJ, Kim YK, Lee MH, Lee JH. Validation of diagnostic criteria using gadoxetic acid-enhanced and diffusion-weighted MR imaging for small hepatocellular carcinoma (<= 2.0 cm) in patients with hepatitis-induced liver cirrhosis. Acta Radiol. 2013; 54:127–136. PMID: 23148300.
81. Shindoh J, Andreou A, Aloia TA, Zimmitti G, Lauwers GY, Laurent A, et al. Microvascular invasion does not predict long-term survival in hepatocellular carcinoma up to 2 cm: reappraisal of the staging system for solitary tumors. Ann Surg Oncol. 2013; 20:1223–1229. PMID: 23179993.
82. Hwang S, Lee YJ, Kim KH, Ahn CS, Moon DB, Ha TY, et al. The impact of tumor size on long-term survival outcomes after resection of solitary hepatocellular carcinoma: single-institution experience with 2558 patients. J Gastrointest Surg. 2015; 19:1281–1290. PMID: 25956724.

83. Sangiovanni A, Manini MA, Iavarone M, Romeo R, Forzenigo LV, Fraquelli M, et al. The diagnostic and economic impact of contrast imaging techniques in the diagnosis of small hepatocellular carcinoma in cirrhosis. Gut. 2010; 59:638–644. PMID: 19951909.

84. Khalili K, Kim TK, Jang HJ, Haider MA, Khan L, Guindi M, et al. Optimization of imaging diagnosis of 1–2 cm hepatocellular carcinoma: an analysis of diagnostic performance and resource utilization. J Hepatol. 2011; 54:723–728. PMID: 21156219.
85. Wu LM, Xu JR, Gu HY, Hua J, Chen J, Zhu J, et al. Is liver-specific gadoxetic acid-enhanced magnetic resonance imaging a reliable tool for detection of hepatocellular carcinoma in patients with chronic liver disease? Dig Dis Sci. 2013; 58:3313–3325. PMID: 23884757.

86. Kitao A, Matsui O, Yoneda N, Kozaka K, Shinmura R, Koda W, et al. The uptake transporter OATP8 expression decreases during multistep hepatocarcinogenesis: correlation with gadoxetic acid enhanced MR imaging. Eur Radiol. 2011; 21:2056–2066. PMID: 21626360.

87. Choi JY, Lee HC, Yim JH, Shim JH, Lim YS, Shin YM, et al. Focal nodular hyperplasia or focal nodular hyperplasia-like lesions of the liver: a special emphasis on diagnosis. J Gastroenterol Hepatol. 2011; 26:1004–1009. PMID: 21251063.

88. Kogita S, Imai Y, Okada M, Kim T, Onishi H, Takamura M, et al. Gd-EOB-DTPA-enhanced magnetic resonance images of hepatocellular carcinoma: correlation with histological grading and portal blood flow. Eur Radiol. 2010; 20:2405–2413. PMID: 20490505.

89. Yoon JH, Lee JM, Yang HK, Lee KB, Jang JJ, Han JK, et al. Non-hypervascular hypointense nodules ≥ 1 cm on the hepatobiliary phase of gadoxetic acid-enhanced magnetic resonance imaging in cirrhotic livers. Dig Dis. 2014; 32:678–689. PMID: 25376284.
90. Golfieri R, Grazioli L, Orlando E, Dormi A, Lucidi V, Corcioni B, et al. Which is the best MRI marker of malignancy for atypical cirrhotic nodules: hypointensity in hepatobiliary phase alone or combined with other features? Classification after Gd-EOB-DTPA administration. J Magn Reson Imaging. 2012; 36:648–657. PMID: 22592930.

91. Akai H, Matsuda I, Kiryu S, Tajima T, Takao H, Watanabe Y, et al. Fate of hypointense lesions on Gd-EOB-DTPA-enhanced magnetic resonance imaging. Eur J Radiol. 2012; 81:2973–2977. PMID: 22280873.

92. Motosugi U. Hypovascular hypointense nodules on hepatocyte phase gadoxetic acid-enhanced MR images: too early or too progressed to determine hypervascularity. Radiology. 2013; 267:317–318.

93. Ichikawa S, Ichikawa T, Motosugi U, Sano K, Morisaka H, Enomoto N, et al. Presence of a hypovascular hepatic nodule showing hypointensity on hepatocyte-phase image is a risk factor for hypervascular hepatocellular carcinoma. J Magn Reson Imaging. 2014; 39:293–297. PMID: 23633285.

94. Kim YK, Lee WJ, Park MJ, Kim SH, Rhim H, Choi D. Hypovascular hypointense nodules on hepatobiliary phase gadoxetic acid-enhanced MR images in patients with cirrhosis: potential of DW imaging in predicting progression to hypervascular HCC. Radiology. 2012; 265:104–114. PMID: 22891358.

95. Yamamoto A, Ito K, Tamada T, Higaki A, Kanki A, Sato T, et al. Newly developed hypervascular hepatocellular carcinoma during follow-up periods in patients with chronic liver disease: observation in serial gadoxetic acid-enhanced MRI. AJR Am J Roentgenol. 2013; 200:1254–1260. PMID: 23701061.

96. Lee DH, Lee JM, Lee JY, Kim SH, Kim JH, Yoon JH, et al. Non-hypervascular hepatobiliary phase hypointense nodules on gadoxetic acid-enhanced MRI: risk of HCC recurrence after radiofrequency ablation. J Hepatol. 2015; 62:1122–1130. PMID: 25529623.

97. Toyoda H, Kumada T, Tada T, Sone Y, Maeda A, Kaneoka Y. Non-hypervascular hypointense nodules on Gd-EOB-DTPA-enhanced MRI as a predictor of outcomes for early-stage HCC. Hepatol Int. 2015; 9:84–92. PMID: 25788383.

98. Merkle EM, Zech CJ, Bartolozzi C, Bashir MR, Ba-Ssalamah A, Huppertz A, et al. Consensus report from the 7th international forum for liver magnetic resonance imaging. Eur Radiol. 2016; 26:674–682. PMID: 26070500.

99. Motosugi U, Bannas P, Sano K, Reeder SB. Hepatobiliary MR contrast agents in hypovascular hepatocellular carcinoma. J Magn Reson Imaging. 2015; 41:251–265. PMID: 25104398.

100. Choi BI, Lee JM, Kim TK, Dioguardi Burgio M, Vilgrain V. Diagnosing borderline hepatic nodules in hepatocarcinogenesis: imaging performance. AJR Am J Roentgenol. 2015; 205:10–21. PMID: 26102378.

101. Golfieri R, Renzulli M, Lucidi V, Corcioni B, Trevisani F, Bolondi L. Contribution of the hepatobiliary phase of Gd-EOB-DTPA-enhanced MRI to dynamic MRI in the detection of hypovascular small (≤ 2 cm) HCC in cirrhosis. Eur Radiol. 2011; 21:1233–1242. PMID: 21293864.
102. Hwang J, Kim YK, Jeong WK, Choi D, Rhim H, Lee WJ. Nonhypervascular hypointense nodules at gadoxetic acid-enhanced MR imaging in chronic liver disease: diffusion-weighted imaging for characterization. Radiology. 2015; 276:137–146. PMID: 25734551.

103. Xu PJ, Yan FH, Wang JH, Shan Y, Ji Y, Chen CZ. Contribution of diffusion-weighted magnetic resonance imaging in the characterization of hepatocellular carcinomas and dysplastic nodules in cirrhotic liver. J Comput Assist Tomogr. 2010; 34:506–512. PMID: 20657216.

104. Hyodo T, Murakami T, Imai Y, Okada M, Hori M, Kagawa Y, et al. Hypovascular nodules in patients with chronic liver disease: risk factors for development of hypervascular hepatocellular carcinoma. Radiology. 2013; 266:480–490. PMID: 23362095.

105. Takechi M, Tsuda T, Yoshioka S, Murata S, Tanaka H, Hirooka M, et al. Risk of hypervascularization in small hypovascular hepatic nodules showing hypointense in the hepatobiliary phase of gadoxetic acid-enhanced MRI in patients with chronic liver disease. Jpn J Radiol. 2012; 30:743–751. PMID: 23001373.

106. Takayama Y, Nishie A, Nakayama T, Asayama Y, Ishigami K, Kakihara D, et al. Hypovascular hepatic nodule showing hypointensity in the hepatobiliary phase of gadoxetic acid-enhanced MRI in patients with chronic liver disease: prediction of malignant transformation. Eur J Radiol. 2012; 81:3072–3078. PMID: 22673776.

107. Di Pietropaolo M, Briani C, Federici GF, Marignani M, Begini P, Delle Fave G, et al. Comparison of diffusion-weighted imaging and gadoxetic acid-enhanced MR images in the evaluation of hepatocellular carcinoma and hypovascular hepatocellular nodules. Clin Imaging. 2015; 39:468–475. PMID: 25748089.

108. Midorikawa Y, Takayama T, Nara S, Hashimoto T, Omichi K, Ebisawa K, et al. No need of immediate treatment for hypovascular tumors associated with hepatocellular carcinoma. World J Surg. 2016; 40:2460–2465. PMID: 27142625.

109. Matsuda M, Ichikawa T, Amemiya H, Maki A, Watanabe M, Kawaida H, et al. Preoperative gadoxetic acid-enhanced MRI and simultaneous treatment of early hepatocellular carcinoma prolonged recurrence-free survival of progressed hepatocellular carcinoma patients after hepatic resection. HPB Surg. 2014; 2014:641685. PMID: 24701029.

110. Midorikawa Y, Takayama T, Shimada K, Nakayama H, Higaki T, Moriguchi M, et al. Marginal survival benefit in the treatment of early hepatocellular carcinoma. J Hepatol. 2013; 58:306–311. PMID: 23063418.

111. An C, Rhee H, Han K, Choi JY, Park YN, Park MS, et al. Added value of smooth hypointense rim in the hepatobiliary phase of gadoxetic acid-enhanced MRI in identifying tumour capsule and diagnosing hepatocellular carcinoma. Eur Radiol. 2016; 10. 21. [Epup]. DOI: 10.1007/s00330-016-4634-6.

112. Galea N, Cantisani V, Taouli B. Liver lesion detection and characterization: role of diffusion-weighted imaging. J Magn Reson Imaging. 2013; 37:1260–1276. PMID: 23712841.

Fig. 1
HCC in 56-year-old man.
A. Pre-contrast T1-weighted image shows hyperintense nodule (arrow) in segment VI of liver. B. In arterial-phase of gadoxetic acid-enhanced magnetic resonance imaging, nodule (arrow) exhibits hyperintensity relative to surrounding liver parenchyma. C. Subtraction image obtained by subtracting pre-contrast-enhanced and arterial-phase T1-weighted images depicts true arterial enhancement of nodule (arrow). HCC = hepatocellular carcinoma
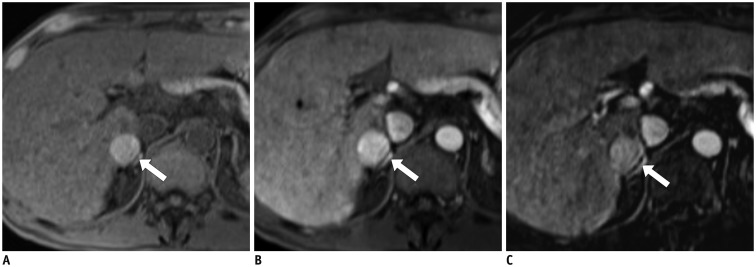
Fig. 2
HCC in 82-year-old man with chronic hepatitis C.
A-C. In gadoxetic acid-enhanced magnetic resonance images, 5-cm mass (arrows) exhibits arterial hyperenhancement (A), slight hyperintensity in portal venous phase (PVP) (B), and hypointensity in transitional phase (C). Washout appearance of nodule in PVP only might lead to false-negative diagnosis of HCC based on enhancement pattern. D. It (arrow) shows hypointensity on HBP. HBP = hepatobiliary phase, HCC = hepatocellular carcinoma
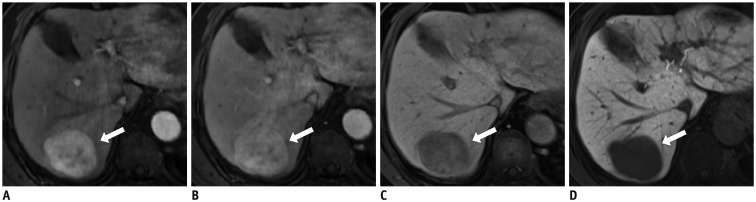
Fig. 3
Sub-centimeter-sized HCC in 56-year-old man with chronic hepatitis B.
Gadoxetic acid-enhanced MR image demonstrates 0.8-cm nodule (arrows) in right lobe of liver, adjacent to portal vein (A). Nodule exhibits arterial hyperenhancement, persistent hyperintensity during portal venous phase (B), and hypointensity during transitional (C), and hepatobiliary phases (D). Lesion (arrows) also exhibits other ancillary features, including intermediate hyperintensity on T2-weighted images (E), and restricted diffusion (F). Lesion was pathologically confirmed as HCC after hepatic resection. HCC = hepatocellular carcinoma
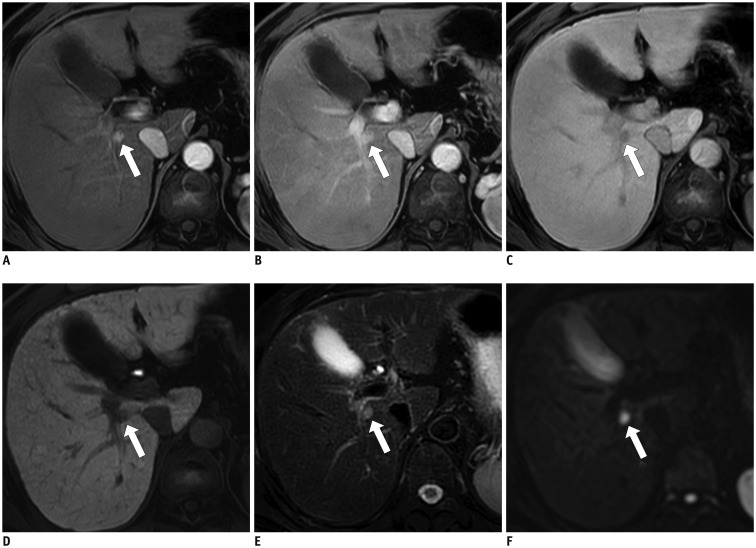
Fig. 4
HCC with hepatobiliary phase (HBP) capsule appearance in 59-year-old female hepatitis B virus carrier.
A. 2.8-cm tumor (arrow) in right posterior hepatic section shows hyperenhancement in arterial phase. B-D. Tumor (arrows) becomes hypointense relative to liver from portal phase (B), to late portal phase (C), and to transitional phase (3 minutes) (D), and shows no conventional capsule appearance (peripheral rim of smooth hyperenhancement). Note that smooth hypointense rim (arrow) begins to appear in transitional phase. E. In HBP, smooth hypointense rim (arrow) clearly surrounds tumor. F. Surgical specimen revealed well-capsulated tumor (cut in half), which was confirmed as HCC with complete fibrous capsule on microscopic examination. HCC = hepatocellular carcinoma
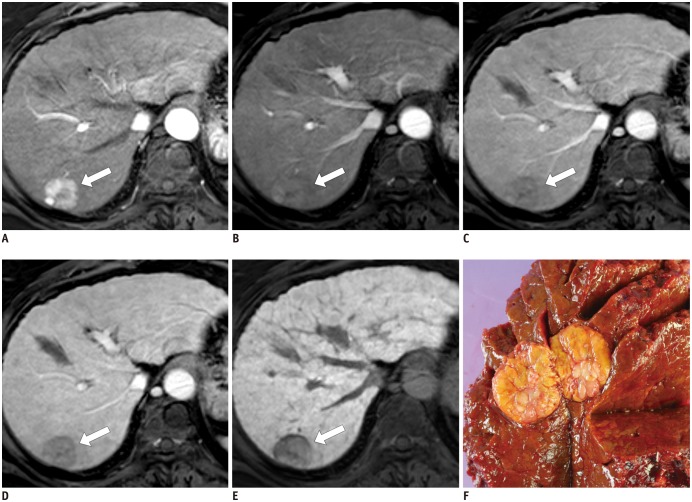
Fig. 5
Moderately differentiated HCC in 60-year-old man.
2.1-cm-sized small hepatic nodule shows (arrows) isointensity during unenhanced T1-weighted image (A), arterial-phase (B), on portal venous and 3 minutes transitional-phase image (not shown) (C), and 20 minutes hepatobiliary-phase images after administration of gadoxetic acid (D). This lesion (arrows) is seen as hyperintense on single-shot echo-planar diffusion-weighed imaging at b = 800 sec/mm2
(E) and T2-weighted image (F). Surgical specimen revealed 2-cm, single nodular type HCC with Edmondson grade II (G). HCC = hepatocellular carcinoma
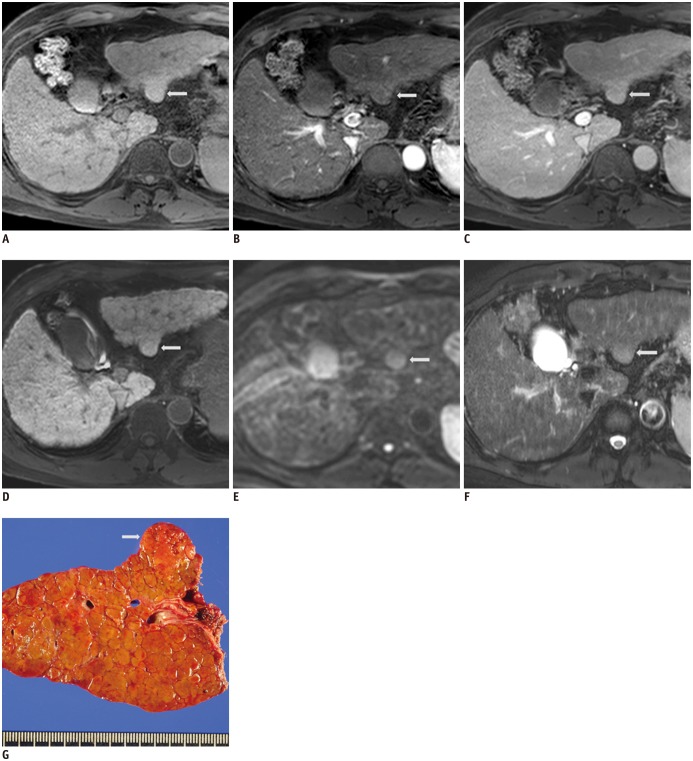
Table 1
Consensus Statements




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download