Abstract
Meningitis is a common central nervous system (CNS) complication of the mumps, a viral infection, but encephalitis and meningoencephalitis are less common in mumps. We describe magnetic resonance imaging findings of acute mumps meningoencephalitis in a 32-year-old male who showed bilateral hippocampal lesions without preceding parotitis. Although it is rare, hippocampal involvement should be considered a CNS complication of mumps infection.
Mumps, also known as epidemic parotitis, is a viral disease caused by the mumps virus. The virus usually causes parotitis but sometimes results in central nervous system (CNS) complications such as acute aseptic meningitis or acute or chronic encephalitis. CNS involvement has been reported to occur in up to 65% of cases of mumps (12), and meningitis accounts for a major proportion of CNS complications (1). The development of encephalitis is rare (3), with a reported incidence of 1 per 6000 mumps cases. However, encephalitis has a much graver prognosis 382than meningitis (12), with mortality rates ranging from 2.3% to 22% and long-term morbidity ranges of from 24% to 33% (1). Here, we describe an adult case of acute mumps meningoencephalitis that manifested as seizure and impaired consciousness with neuroimaging findings of bilateral hippocampal encephalitis and meningitis. The patient had no preceding clinical symptoms of acute parotitis, which made an early diagnosis of mumps difficult.
Institutional Review Board approval was obtained, and the requirement for informed consent was waived due to the retrospective nature of this case report. A 32-year-old man was brought to the emergency room of our hospital with sudden-onset generalized seizures, which developed suddenly and stopped spontaneously after about three minutes without the administration of anticonvulsants; post-ictal stuporous mentality followed. The patient had suffered from headaches and fever and a sensation of chills for six days prior to his hospital visit. He had no medical history of note and was admitted to our neurology department.
Neurologic examination at admission revealed impaired orientation and cognition with decreased attention. He responded only to painful stimulation and presented with aphasia. Spontaneous eye opening was possible, and there was no sensory or motor impairment. Clinical signs of meningitis, including nuchal rigidity and Kernig and Brudzinski signs, were observed. At this time, his temperature and blood pressure were 38.5℃ and 136/56 mm Hg, respectively. His laboratory peripheral blood results revealed lymphocyte-predominant leukocytosis (white blood cell count, 16760/µL; lymphocytes, 67.2%), and a cerebrospinal fluid (CSF) examination revealed no remarkable findings. Antibodies for cytomegalovirus and orgherpes simplex virus were negative, and microorganisms including group B Streptococcus, Enterovirus, and Mycobacterium tuberculosis were not found on CSF examination; a serologic test for Japanese encephalitis antibody was also negative. Initial brain CT and brain magnetic resonance imaging (MRI) findings were normal. However, initial electroencephalography revealed continuous, irregular, slowing background activity and many spikes, waves, and bursts of semi-rhythmic delta activity in the bilateral fronto-temporal areas that suggested diffuse cerebral dysfunction. Phenytoin was administered to control seizures, but they reoccurred nevertheless. On admission day 3, a laboratory test was performed for mumps infection; the patient's serum mumps immunoglobulin M (IgM) titer was 2.6 (normal range, < 0.8), but his immunoglobulin G (IgG) was equivocal (titer, 0.86; equivocal for 0.8–1.2). Although we could not establish our patient's vaccination history, these findings suggested active mumps infection, and we diagnosed mumps meningoencephalitis based on the patient's serological and brain imaging findings; notably, no symptoms of mumps infection (such as parotitis or orchitis) other than symptoms of meningoencephalitis were found. We started the patient on empirical intravenous acyclovir treatment.
However, on admission day 7, his consciousness worsened to a deep stupor, and respiration became sufficiently unstable that the patient required endotracheal intubation and artificial ventilation. Follow-up CSF examination revealed lymphocyte-predominant pleocytosis (18 nucleated cells/mm2 [74% lymphocytes]). On the same day, T2-weighted and diffusion-weighted brain MRI images revealed increased signal intensity areas in the bilateral hippocampi, and postcontrast T1-weighted images revealed diffuse leptomeningeal enhancement but no contrast enhancement of the bilateral hippocampal lesions (Fig. 1).
On admission day 10, the patient became afebrile and alert, although intermittent seizure attacks persisted. Follow-up serology testing showed that his mumps IgM was negative (titer, 0.49), which suggested treatment response, but that IgG remained equivocal (titer, 0.83). The patient's brain MRI on admission day 20 showed improvement of the bilateral hippocampal lesions and the disappearance of meningeal enhancement (Fig. 2), and subsequently, his clinical condition improved steadily. Complete recovery of neurologic symptoms was noted on admission day 36, and the patient was discharged.
Herein, we report a unique case of mumps meningoencephalitis in an adult that involved the bilateral hippocampi without preceding parotitis. Bilateral hippocampal encephalitis and meningitis were observed by brain MRI, and mumps was diagnosed based on elevated mumps-specific IgM serum titers. We consider that this case was a pure form of mumps meningoencephalitis, which is an extremely uncommon form of mumps. Fortunately, the patient's neurologic symptoms fully abated, and follow-up serologic testing revealed negative conversion of mumps-specific serum IgM, which suggested a treatment response.
Symptoms of mumps are generally mild–moderately elevated body temperature and parotid swelling (1). However, serious complications such as orchitis, pancreatitis, meningitis, and encephalitis occasionally occur (1). At most 10% of all patients with mumps parotitis develop clinical meningitis (45), which may develop without parotid gland swelling, as occurred in the present case. Mumps encephalitis is uncommon in adults (3), but when it is encountered, impaired consciousness, seizures, and psychological symptoms are often observed; in such cases, serological diagnosis or virus isolation is required for differential diagnosis. Furthermore, mumps may be infrequently complicated by acute encephalitis, which is generally mild and shows no focal signs of meningeal irritation (3).
Two pathological mechanisms have been proposed for mumps encephalitis (26). The first, primary mumps meningoencephalitis, involves direct invasion of the CNS by the virus and is associated with the appearance of encephalitis during the early disease stage. Furthermore, autopsy findings of diffuse edema, perivascular cuffing, cytolysis, and satellitosis without evidence of demyelination have been ascribed to primary mumps meningoencephalitis (6). The second type results from immune-mediated demyelination, which occurs on average 10 days after disease onset. Acute disseminated encephalomyelitis (ADEM) and clinically mild encephalitis with reversible splenial lesions are included in this type (6). We consider that the meningoencephalitis in our case was caused by direct viral invasion rather than an autoimmune process because neurological abnormalities occurred on day six after disease onset and no white matter abnormalities were visualized on MRI. However, direct viral infiltration of the CNS was not confirmed by mumps virus mRNA reverse transcription polymerase chain reaction (RT-PCR).
Focal CNS involvement is less common in mumps than is meningitis. In the English literature, mumps encephalitis affects the brainstem, basal ganglia, splenium of the corpus callosum, and white matter, and it is considered a form of ADEM (12345678910); however, bilateral hippocampal lesions resulting from mumps viral infection have not been previously reported. Other pathogens that should be included in the differential diagnosis of bilateral hippocampal lesions include herpes simplex virus, Ebstein-Barr virus, and Cytomegalovirus. In the case described here, these pathogens were ruled out by CSF findings. Focal cerebral edema secondary to persistent temporal lobe seizures might be considered imaging findings in our case, but clinical course and serological evidence of apparent mumps infection strongly suggested that the patient's condition resulted from the mumps. Notably, no established effective therapy is available for mumps encephalitis (2), although vaccination provides useful prophylaxis (2). We could not establish a vaccination history for our patient.
Early accurate diagnosis is important, but in the present case, mumps infection was not easily detected. In its early stage, mumps meningoencephalitis may clinically mimic other acute brain disorders such as pyogenic brain abscess, acute multiple sclerosis, tuberculous meningitis, or drug intoxication (1), and radiologic findings are either normal or nonspecific. In our patient, clinical symptoms or signs (such as parotid swelling) that readily suggest mumps infection were absent. Mumps meningitis occurs in the absence of salivary gland involvement in up to 50% of cases, and it can precede salivary symptoms or occur after the appearance of parotitis (5). According to studies published in the 1950s and 1960s, the incidence of meningoencephalitis without salivary gland involvement was 43–49% in epidemic mumps regions (10). One case report does describe brain stem involvement on a brain MRI in an adult case of mumps encephalitis without salivary gland involvement (2). The rate of mumps viral infection has been greatly decreased by vaccination, and as a result, clinical suspicion of mumps meningoencephalitis is difficult, especially when there is no salivary gland involvement.
Our patient only had symptoms of CNS infection and abnormal neuroimaging findings, and it was immunoassays of serum that led to a diagnosis of acute mumps infection. Although brain parenchymal involvement in the mumps is extremely rare, symmetric hippocampal lesions could be considered when other pathogens of CNS infection are not evident on laboratory examination.
We here described unique neuroimaging findings of mumps meningoencephalitis that affected the bilateral hippocampi. Although it is rare, mumps meningoencephalitis should be considered in the differential diagnosis of bilateral hippocampal lesions and in the presence of evidence of an infectious CNS disorder.
Figures and Tables
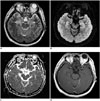 | Fig. 1Brain MRI taken 7 days after seizure onset in 32-year-old male patient.Axial T2-weighted image (A) and diffusion-weighted image (B) showing high signal intensities in bilateral hippocampi (arrows) but no significant abnormality on corresponding apparent diffusion coefficient map (C). Axial post-contrast T1-weighted image (D) showing diffuse leptomeningeal enhancement and no enhancement of bilateral hippocampal lesions.
|
References
1. Tarr RW, Edwards KM, Kessler RM, Kulkarni MV. MRI of mumps encephalitis: comparison with CT evaluation. Pediatr Radiol. 1987; 17:59–62.
2. Koyama S, Morita K, Yamaguchi S, Fujikane T, Sasaki N, Aizawa H, et al. An adult case of mumps brainstem encephalitis. Intern Med. 2000; 39:499–502.
3. Alfaro A, Granda D. [Mumps encephalitis in adulthood]. Neurologia. 1991; 6:108–110.
4. Unal A, Emre U, Atasoy HT, Sumer MM, Mahmutyazicioglu K. Encephalomyelitis following mumps. Spinal Cord. 2005; 43:441–444.
5. Hviid A, Rubin S, Mühlemann K. Mumps. Lancet. 2008; 371:932–944.
6. Taylor FB Jr, Toreson WE. Primary mumps meningo-encephalitis. Arch Intern Med. 1963; 112:216–221.
7. Suga K, Goji A, Shono M, Matsuura S, Inoue M, Toda E, et al. Mumps encephalitis with akinesia and mutism. Pediatr Int. 2015; 57:721–724.
8. Kimura K, Fuchigami T, Ishii W, Imai Y, Tanabe S, Kuwabara R, et al. Mumps-virus-associated clinically mild encephalopathy with a reversible splenial lesion. Int J Clin Pediatr. 2012; 1:124–128.
9. Sirmatel O, Yazgan P, Sirmatel F, Ozturk A, Ziylan Z. Radiological findings in a mumps case with multiple complications. Indian J Radiol Imaging. 2006; 16:305–308.
10. Murray HG, Field CM, McLeod WJ. Mumps meningoencephalitis. Br Med J. 1960; 1:1850–1853.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download