Abstract
Ultrasound-guided core needle biopsy (US-CNB) is an important step in the diagnosis of musculoskeletal soft-tissue lesions. To maximize efficacy and minimize the complications of US-CNB, it is critical to collaborate with a multidisciplinary team and to understand the particular considerations of US-CNB for these lesions. The purpose of this article is to provide a systematic review and step-by-step tips for using US-CNB to diagnose musculoskeletal soft-tissue lesions.
Biopsy is a key step in the diagnosis and management of musculoskeletal soft-tissue lesions, even though not all lesions require a biopsy; biopsy is generally recommended for a lesion when its clinical and radiologic features do not suggest a typically benign entity or when it is detected in patients with a history of malignancy. Although surgical excisional biopsy is still considered the gold standard for the ultimate diagnosis, there is a general trend toward using minimally invasive procedures, and percutaneous core needle biopsy (CNB) is gaining favor over surgical excisional biopsy for the initial tissue diagnosis of musculoskeletal soft-tissue lesions (1). In particular, ultrasonography (US) is a guidance system that is most commonly used for obtaining biopsy materials from musculoskeletal soft-tissue lesions because it has many advantages: it is easy to handle; it is usually quicker than any other guidance modality; it provides real-time, multi-planar imaging; and it avoids subjecting the patient to radiation. Performing ultrasound-guided CNB (US-CNB) for musculoskeletal soft-tissue lesions is not much different than non-musculoskeletal lesions, but in some ways, it does require closer collaboration by a multidisciplinary team that consists of a surgical oncologist, medical oncologist, radiation oncologist, and musculoskeletal pathologist with a careful review of all relevant clinical and radiological information. To maximize diagnostic yield and minimize the morbidity of US-CNB, understanding the following considerations is critical: 1) selecting the proper biopsy target in order to yield the most useful specimen; 2) selecting the proper biopsy route in order to avoid damage to important structures and prevent unintended wide excision or local tumor recurrence; and 3) the principles and tips of biopsy techniques. In this article, we hope to provide useful guidelines for applying US-CNB to musculoskeletal soft-tissue lesions.
The first objective of biopsy is to obtain diagnostic material. Because musculoskeletal soft-tissue lesions often have heterogeneous imaging features, it is imperative to target the most diagnostic portion of the lesions. Various image modalities can provide important information that can guide the proper selection of the biopsy site, but in general, magnetic resonance imaging is the modality of choice for characterizing soft-tissue lesions. Intravenous gadolinium is useful for identifying solid or more vascular portions in lesions, and these portions are more likely to yield diagnostic specimens than are cystic or necrotic lesions (2). Positron emission tomography images can help guide biopsy in the target areas, which may result in a higher diagnostic yield by indicating the metabolic activity of a lesion. US is gaining wider acceptance as a suitable biopsy modality for soft-tissue lesions and is considered useful for differentiating solid portions from the cystic or necrotic portions of lesions (3). On US, the solid portion usually demonstrates iso- to hyper-echogenicity; however, in the case of a lesion with hemorrhage or a fatty component, the solid portion could show as a relatively hypoechoic area and thus could be confused with the cystic or necrotic portion. Therefore, it is critical to review all imaging studies and compare their results with the US findings (Figs. 1, 2).
The biopsy must be carefully planned with an understanding of the orthopedic surgeon's perspective, and the optimal biopsy tract should be chosen after considering the location, size, and morphological features of the lesion. It is better to use a shorter path to the lesion, although the shortest distance to the lesion is not necessarily the optimal route (4). The biopsy tract must be placed within the surgical resection margins because the en bloc resection of the tumor, as well as the biopsy tract, is needed to avoid possible tumor seeding along the tract. It is best if the point of needle entry is located along the planned surgery incision (Fig. 3). In addition, the biopsy route should be selected so that the needle does not violate unaffected compartments or neurovascular bundles in order to prevent unintended wide excision, local tumor recurrence, or functional deficits (Figs. 4, 5). Therefore, knowledge of the anatomic compartments and location of the main neurovascular bundle is very important for obtaining a biopsy of any soft-tissue lesions in the extremities.
There are three compartments in the upper leg. The anterior compartment includes the quadriceps muscle group, the iliopsoas, sartorius, and tensor fascia lata muscles, and the iliotibial band. The medial compartment includes the adductor muscle group and the gracilis muscle. The posterior compartment includes the hamstring muscle group (Fig. 6A). There are also three compartments in the lower leg. The anterior compartment includes the tibialis anterior, extensor hallucis longus, and extensor digitorum longus muscles. The lateral compartment includes the peroneus longus and brevis muscles. The posterior compartment can be separated into superficial and deep posterior compartments by the transverse intermuscular septum. The superficial posterior compartment contains the soleus, gastrocnemius, and plantaris muscles. The deep posterior compartment contains the flexor digitorum longus, tibialis posterior, flexor hallucis longus, and popliteus muscles (Fig. 6B). In the upper arm, there are two compartments. The anterior compartment includes the biceps, brachialis, and coracobrachialis muscles, and the posterior compartment includes the triceps muscle (Fig. 6C). The forearm can be described as containing two or three compartments. In the two-compartment description, the anterior or flexor compartment is separated from the posterior or extensor compartment by the radius, ulna, and intermuscular septum. In the three-compartment classification commonly used by surgeons, three muscles–the brachioradialis, extensor carpi radialis longus, and extensor carpi radialis brevis–are considered the lateral compartment (Fig. 6D) (256).
The automated needle uses a two-stage spring-deployed action in which the inner stylet is initially propelled forward to expose the specimen notch, and this is followed by an outer cutting cannula (Fig. 7A). These two stages of the biopsy are powerful and fast, making it useful for obtaining biopsy materials from hard or movable soft-tissue lesions. In contrast, the inner stylet of the semiautomated needle is manually advanced (Fig. 7B). However, this movement lacks both force and speed, and only the movement of the cutting cannula over the inner stylet is automated. The manual advancement of the inner stylet allows for more delicate control, but there is a higher probability of distally displacing the lesion, and thus, the cannula may partially cut the lesion, leaving the remaining core tissue as the only adjacent normal tissue (Fig. 7C). Previously published studies compared the semiautomated and automated core needles for obtaining biopsy materials from breast lesions and reported a better diagnostic yield using automated needles. This is likely because breast lesions are usually mobile and more likely to be displaced distally by the force of the biopsy mechanism, especially when the lesion is small (27).
The introducer–which is first inserted near or inside the edge of the lesion–serves as a sheath and enables the user to obtain multiple core samples. It can also minimize soft-tissue damage and contamination by tumor cells during multiple biopsies.
According to a previous study, there are no differences in diagnostic yield based on needle gauge (8). Generally, a thinner needle is safer and causes less pain, but it is difficult for a thin needle to penetrate a thick or hard-tissue lesion, and thin needles are difficult to control within the tissue. In the case of a calcified or ossified lesion, a larger needle can be more helpful. We suggest an 18-gauge core needle, which is sufficient for most biopsies of musculoskeletal soft-tissue lesions.
There are many kinds of biopsy gun systems, and each system has different lengths for the specimen notch. Wu et al. (8) reported a significant increase in diagnostic yield with longer specimens: only 42% of specimens that were < 5 mm in length were diagnostic, as opposed to 82% of specimens that were > 10 mm. On the basis of these results, attempts should be made to obtain longer specimens if possible.
Wu et al. (8) reported that the cumulative diagnostic yield plateaued at four specimens for musculoskeletal soft-tissue lesions. Other analogous studies on breast lesion biopsies found that four samples were needed to obtain a diagnosis in 100% of biopsies (7). On the basis of these results, we suggest obtaining a minimum of four biopsy specimens for musculoskeletal soft-tissue lesions.
When US-guided biopsy is performed on a soft-tissue lesion, the lateral approach (i.e., the in-plane technique) is generally recommended, and the needle is aligned with the small side of the transducer. As a general rule, a finer needle is more difficult to visualize on US than a thicker needle, whereas a thicker needle is more clearly visible and often causes US reverberation or comet-tail artifacts behind the needle. If the needle gauge is the same, the needle is more visible as the incidence angle of the needle to the US beam increases, and the needle can be optimally visualized when the US beam is perpendicular to it (Fig. 8). The needle's incidence angle can be increased by pressing on the opposite side of the transducer rather than controlling the needle: this technique is called the "heel-and-toe" maneuver. For this technique, it is helpful to use a great deal of gel under one end of the transducer (9).
Ultrasound-guided-core needle biopsy is now increasingly used on small soft-tissue lesions. This is due to technical advances in imaging that allow early detection of small lesions because of high sensitivity. In fact, obtaining biopsy materials from small lesions could be worrisome because targeting small lesions is technically difficult and the obtained specimens are likely to be inadequate, thus hindering accurate diagnosis. However, according to a previous study, performing US-CNB on musculoskeletal soft-tissue lesions is effective for diagnosis and decision-making even if the lesion is < 2 cm (1). Due to the technical aspects of biopsy, as previously mentioned, small, movable soft-tissue lesions are more likely to be displaced distally by the force of the biopsy mechanism. For successful penetration of a lesion with the needle, it is helpful to aim at the center of a lesion rather than the periphery (Figs. 9, 10). It is also important to embed the tip of the introducer needle into the lesion before deploying the inner stylet (7).
1. For optimal visualization of the needle, increase the needle's incidence angle to the US beam.
2. Regarding the gauge of the core biopsy needle, an 18-gauge needle is sufficient in most cases.
3. Try to obtain longer specimens; diagnostic yield increases with longer specimens.
4. Try to obtain ≥ 4 specimens.
5. The heel-and-toe technique is helpful for clearly visualizing the entire needle.
6. Try to aim for the center of lesion if it is small and movable and do not hesitate to biopsy small lesions.
7. Embed the tip of the introducer needle into the lesion before deploying the inner stylet.
Ultrasound-guided-core needle biopsy has become a key step in the diagnosis of musculoskeletal soft-tissue lesions. The goal of any biopsy is to obtain adequate tissue for accurate diagnosis while minimizing morbidity. Here, we have presented the principles and tips for diagnostic and safe US-CNB, and we hope these practical guidelines are helpful for performing successful biopsy.
Figures and Tables
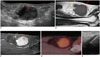 | Fig. 167-year-old female patient with mass in her right posterior thigh.
A. Longitudinal US image showing mass with well-defined, ovoid margins (white arrowheads). Proximal portion of mass (yellow arrow) shows heterogeneous hyper-echogenicity, and distal portion (red arrow) shows homogeneous hypoechogenicity. Deciding proper target is deliberate process for successful US-CNB. B-D. Sagittal T1-weighted MR image (B), enhanced T1-weighted MR image (C), and positron emission tomography (PET) image (D). Proximal portion of mass (yellow arrows) shows high signal intensity with multiple septations on sagittal T1-weighted MR image. This portion does not demonstrate enhancement on enhanced T1-weighted image, thus suggesting fat component. In contrast, distal portion (red arrows) shows homogeneous, intense enhancement on enhanced T1-weighted image and hypermetabolic activity on PET image. When we consider proper targeting, distal portion (red arrow) is best. E. On longitudinal US image, US-CNB was performed at distal hypoechoic area, and this mass was confirmed as well-differentiated liposarcoma. US = ultrasonography, US-CNB = ultrasound-guided core needle biopsy
|
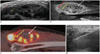 | Fig. 255-year-old female patient with mass in her right anterior thigh.
A. Longitudinal US image shows multi-lobulated mass. Most proximal portion of mass (red arrow) shows homogeneous hypoechogenicity, and just distal portion (yellow arrow) shows heterogeneous hyperechogenecity; lower portion (white arrow) shows homogeneous hyperechogenecity. It was difficult to determine proper target by considering only ultrasonographic appearance. B, C. On sagittal enhanced T1-weighted MR (B) and PET images (C), most proximal portion of mass (red arrows) enhanced well and revealed hypermetabolic activity. Just distal portion (yellow arrows) shows mild enhancement on enhanced T1-weighted image; however, this portion does not show significant metabolic activity on PET image. Lower portion (white arrows) reveals nonenhancing cystic area with hemorrhagic component. Proper target is most proximal portion (red arrow). D. On longitudinal US image, US-CNB was performed at most proximal portion of mass, and this mass was confirmed as undifferentiated pleomorphic sarcoma. PET = positron emission tomography, US = ultrasonography, US-CNB = ultrasound-guided core needle biopsy
|
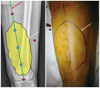 | Fig. 367-year-old female patient with mass in her right posterior thigh.On radiographic image and actual picture of right upper leg, approximate margin of mass is marked with dark line (white arrows), and there are number of predicted entry points for US-CNB (shown as blue and red dots). Blue dots are located within margins of mass and are included on planned incision line for surgery (red arrows); these are proper needle entry points. In contrast, red dots are located outside of margins of mass, and these are poor needle entry points that could lead to unnecessary resection. US-CNB = ultrasound-guided core needle biopsy
|
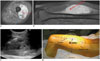 | Fig. 476-year-old male patient with mass in his left lateral thigh.
A, B. On axial (A) and sagittal enhanced T1-weighted images (B), there is heterogeneously enhancing, solid mass that involves vastus intermedius and vastus lateralis muscles. Broadly, two biopsy routes can be considered (as shown by blue and red arrows). Blue route traverses and violates unaffected posterior compartment, but red route crosses only anterior compartment and thus is proper route. C. On longitudinal US image, US-CNB was performed on this mass via red route. D. In actual picture of left upper leg, biopsy entry point was within intended field of surgery and on planned surgery incision line. Imaginary blue biopsy route is marked with blue dots (blue arrow). This mass was confirmed as undifferentiated pleomorphic sarcoma. US = ultrasonography, US-CNB = ultrasound-guided core needle biopsy
|
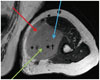 | Fig. 581-year-old female patient with mass in her left upper arm.On axial enhanced T1-weighted image, there is homogeneously enhancing, solid mass that involves biceps brachii muscle. Three biopsy routes can be considered, as shown by blue, green, and red arrows. Blue route crosses uninvolved muscle, and green route could violate median nerve (star) and brachial artery (cross) within mass. Red route does not traverse uninvolved muscular structure and is relatively safe in terms of not violating neurovascular bundle: this is proper route for US-CNB. US-CNB was performed on this mass via red route, and this mass was confirmed as lymphoma. US-CNB = ultrasound-guided core needle biopsy
|
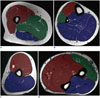 | Fig. 6Compartments of arm and leg.
A. Compartments of upper leg. Anterior compartment (red) includes quadriceps muscle group, iliopsoas, sartorius, and tensor fascia lata muscles, and iliotibial band. Medial compartment (green) includes adductor muscle group and gracilis muscle. Posterior compartment (blue) includes hamstring muscle group. B. Compartments of lower leg. Anterior compartment (red) includes tibialis anterior, extensor hallucis longus, and extensor digitorum longus muscles. Lateral compartment (green) includes peroneus longus and brevis muscles. Posterior compartment (blue) includes soleus, gastrocnemius, plantaris, flexor digitorum longus, tibialis posterior, flexor hallucis longus, and popliteus muscles. Posterior compartment can be separated into superficial and deep compartments by transverse intermuscular septum (white dotted line). C. Compartments of upper arm. Anterior compartment (red) includes biceps, brachialis, and coracobrachialis muscles, and posterior compartment (blue) includes triceps muscle. D. Compartments of forearm. In two-compartment description, anterior or flexor compartment (red) is separated from posterior or extensor compartment (blue) by radius, ulna, and intermuscular septum. Using three-compartment classification, three muscles–brachioradialis, extensor carpi radialis longus, and extensor carpi radialis brevis–are considered lateral compartment (green).
|
 | Fig. 7Two types of core biopsy needle.
A. Automated core biopsy needle (Acecut; TSK Laboratory, Tochigi, Japan) has both single and two-stage firing options. B. Semiautomated biopsy needle (Stericut; TSK Laboratory). Inner needle of this biopsy system can be introduced by hand for added safety and tactile sensitivity, and needle can be delivered in combination with coaxial introducer needle. C. Manual advancement of inner stylet of semiautomated biopsy needle allows for more delicate control; however, there is higher probability of distally displacing lesion, and thus cannula may partially cut lesion; as result, rest of core tissue would be only adjacent normal tissue.
|
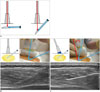 | Fig. 8Conceptual diagram for ultrasonographic visualization of biopsy needle.
A. Biopsy needle is better visualized when US beam is steered as close to perpendicular to needle as possible. B. Visualization becomes poor at steep insertion angles because echoes are reflected away from the transducer. C. Heel-and-toe maneuver is performed to bring transducer face into parallel arrangement with needle shaft. US beam will hit perpendicular to needle shaft, thus producing optimal visualization. US = ultrasonography
|
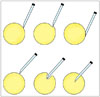 | Fig. 9Conceptual diagram for US-CNB for small soft tissue lesion.Traversing lesion with core needle may be difficult when lesion is small and mobile. Aiming at center of lesion rather than periphery is helpful for traversing it. US-CNB = ultrasound-guided core needle biopsy
|
 | Fig. 1056-year-old female patient with soft-tissue mass in her left knee.
A, B. On axial enhanced T1-weighted (A) and sagittal T2-weighted MR images (B), there is ovoid mass in subcutaneous fat layer of lateral aspect of left knee. This lesion was movable and measured < 0.6 cm in diameter. C. On longitudinal US image, US-CNB was successfully performed via center of lesion, and this lesion was confirmed as myopericytoma. US = ultrasonography, US-CNB = ultrasound-guided core needle biopsy
|
References
1. Kim SY, Chung HW. Small musculoskeletal soft-tissue lesions: US-guided core needle biopsy--comparative study of diagnostic yields according to lesion size. Radiology. 2016; 278:156–163.
2. Huang AJ, Kattapuram SV. Musculoskeletal neoplasms: biopsy and intervention. Radiol Clin North Am. 2011; 49:1287–1305. vii
3. Balach T, Stacy GS, Haydon RC. The clinical evaluation of soft tissue tumors. Radiol Clin North Am. 2011; 49:1185–1196. vi
4. Mavrogenis AF, Angelini A, Errani C, Rimondi E. How should musculoskeletal biopsies be performed? Orthopedics. 2014; 37:585–588.
5. Toomayan GA, Robertson F, Major NM. Lower extremity compartmental anatomy: clinical relevance to radiologists. Skeletal Radiol. 2005; 34:307–313.
6. Toomayan GA, Robertson F, Major NM, Brigman BE. Upper extremity compartmental anatomy: clinical relevance to radiologists. Skeletal Radiol. 2006; 35:195–201.
7. Sridharan R, Yunos SM, Aziz S, Hussain RI, Alhabshi SM, Suria Hayati MP, et al. Comparison on the use of semi-automated and automated core biopsy needle in ultrasound guided breast biopsy. Med J Malaysia. 2015; 70:326–333.
8. Wu JS, Goldsmith JD, Horwich PJ, Shetty SK, Hochman MG. Bone and soft-tissue lesions: what factors affect diagnostic yield of image-guided core-needle biopsy? Radiology. 2008; 248:962–970.
9. Song HS, Kim DY, Yoon KS. Intervention using ultrasonography. J Korean Orthop Assoc. 2013; 48:342–349.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download