Abstract
Objective
To evaluate the in vivo efficiency of the biopsy tract radiofrequency ablation for hemostasis after core biopsy of the liver in a porcine liver model, including situations with bleeding tendency and a larger (16-gauge) core needle.
Materials and Methods
A preliminary study was performed using one pig to determine optimal ablation parameters. For the main experiment, four pigs were assigned to different groups according to heparinization use and biopsy needle caliber. In each pig, 14 control (without tract ablation) and 14 experimental (tract ablation) ultrasound-guided core biopsies were performed using either an 18- or 16-gauge needle. Post-biopsy bleeding amounts were measured by soaking up the blood for five minutes. The results were compared using the Mann-Whitney U test.
Results
The optimal parameters for biopsy tract ablation were determined as a 2-cm active tip electrode set at 40-watt with a tip temperature of 70–80℃. The bleeding amounts in all experimental groups were smaller than those in the controls; however they were significant in the non-heparinized pig biopsied with an 18-gauge needle and in two heparinized pigs (p < 0.001). In the heparinized pigs, the mean blood loss in the experimental group was 3.5% and 13.5% of the controls biopsied with an 18- and 16-gauge needle, respectively.
Recently, important improvements in the era of diagnostic imaging technology have been made. However, percutaneous core liver biopsy is still used as a confirmative diagnostic method of diffuse liver disease, as well as focal liver lesions (1). In general, more accurate and reliable the pathology results are obtained with more biopsy specimens of larger size (23). However, the size and the number of biopsy specimens to be taken are limited because of the concern for bleeding complication, especially for patients with bleeding tendencies (coagulation defects, decreased platelet counts, or large amounts of perihepatic ascites and etc.).
Many efforts have been made to prevent bleeding complications after hepatic core needle biopsy. These included 1) biopsy through a transjugular route (4); 2) embolization of endogenous or exogenous materials along biopsy tract (5678910); and 3) thermal ablation of biopsy tract using radiofrequency (RF) energy (111213). Several experimental studies of RF ablation have demonstrated promising results of post-biopsy bleeding control (11121314). However, clinical adoption of these techniques has been slow, perhaps because the devices including RF electrode used are mostly designed for only experimental aim and are limited to use in clinical settings.
This study was based on our 10 years of experience performing percutaneous ultrasound (US)-guided RF ablation procedures for hepatocellular carcinoma with the assistance of artificial ascites. Our earlier studies showed that sufficient coagulation of the RF electrode path would minimize the possibility of bleeding, even in patients with artificially infused ascites (15). Currently, large amount of ascites is no longer a contraindication for percutaneous RF ablation for hepatic tumors in our institution.
A new RF device for coagulating the biopsy tract is the collective result of previous studies. The combination of insulation sheath and biopsy needle can be used in a coaxial manner during biopsy. Monitoring the temperature of electrode tip using thermocouple facilitates appropriate hemostasis (12). It is also compatible with a commercially-available biopsy needle and an RF generator. Thus, the purpose of the present study was to demonstrate, in an in vivo porcine model, the pre-clinical efficacy of the biopsy tract ablating device for producing hemostasis after core biopsy of the liver, even in the situations with bleeding tendency and when using a 16- and 18-gauge core needle.
This study protocol was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC No. 20130311001) of the Samsung Biomedical Research Institute. All applicable institutional and/or national guidelines for the care and use of animals were followed. Five female domestic pigs weighing approximately 60 kg each were included as study animals. One pig was used for preliminary study and the others were allocated into one of two groups i.e., non-heparinized group (n = 2), or heparinized group (n = 2).
Each pig was anesthetized with an intramuscular injection of 5 mg/kg of zolazepam-tiletamine (Zoletil; Virbac Laboratories, Carros, France) and 0.5 mg/kg of xylazine hydrochloride (Rompun; Bayer, Leverkusen, Germany). Endotracheal intubation was performed. Anesthesia was maintained with mechanical ventilation using inhaled isoflurane gas (Forane; JW Pharmaceuticals, Seoul, Korea) at a 2% concentration for adequate effect. All pigs were placed in a supine position and a laparotomy was performed to expose the livers through a subcostal incision. Two ground pads connected to a generator were attached bilaterally at the hips before the start of the experiment.
Blood samplings were performed once before the experiment in the non-heparinized group. In the heparinized group, a heparin bolus of 300 U/kg was administered intravenously just after laparotomy (1214). Blood samples were taken before and after heparin administration. A total of six samples were tested for red-blood cell count, hemoglobin level, hematocrit level, platelet count, prothrombin time, and activated partial thromboplastin time (aPTT).
The needle tract ablator made from the company (STARmed, Goyang, Korea) was composed of two main parts including an insulation sheath and an electrode connected to a multi cable (Fig. 1). The insulation sheath was made of stainless steel and coated with a polyester film marked 1-cm interval. The insulation sheath was 13-cm long. The outer diameter of the insulation sheath was either 17-gauge for an 18-gauge biopsy needle or 15-gauge for a 16-gauge biopsy needle. It can be used as both a trocar during biopsy and an insulation sheath during traction ablation.
The electrode was made of stainless steel without coating. The electrode was 14-cm or 15-cm long and the active tip length was 1 cm or 2 cm when combined with the insulation sheath. A thermocouple was embedded within the tip of electrode. The multi cable with the connector, which is available for delivering energy and receiving thermal information, connects the electrode to the generator.
A preliminary study was performed with one pig to determine adequate parameters for tract ablation, including active tip length, ablation power (W), and temperature of electrode tip. After acquiring one core needle biopsy specimen, we tested under various combinations at the following settings: electrodes with 1- and 2-cm active tip lengths and ablation power ranging from 10–40 W. We performed all the tract ablation keeping the temperature of the electrode tip within the range of 70–80℃ to avoid insufficient ablation or causing immediate excessive collateral tissue damage, as established in a previous study (12). The preliminary study established ideal parameters for bleeding control by visual inspection without quantitation.
Four pigs were assigned to each category according to whether they underwent heparinization and the caliber of the biopsy needle, either an 18- or 16-gauge. In each pig, 28 US-guided core-biopsies were performed under control (no tract ablation, n = 14) and experimental (tract ablation, n = 14) settings.
One of the authors (with > 7 years' experience in abdominal imaging and US-guided interventional procedures) performed all biopsies using an automatic side-cutting core biopsy needle (ACECUT; TSK Laboratory, Tochigi, Japan). All biopsy procedures were performed under US-guidance (Accuvix A30; Samsung Medison, Seoul, Korea) to make ensure no large vessels or previous biopsy tract along the current biopsy needle path that would affect the results (Fig. 2A). Control and experimental biopsies were coupled, so that after a control biopsy, the corresponding experimental biopsy was performed close to the control puncture site with tract ablation, in order to avoid location and vascularity bias. A combination of biopsy gun and an insulation sheath were inserted 3 cm into the liver (Fig. 2B). The depth of biopsy gun from the liver surface was checked by reading the marking on an insulation sheath; the biopsy gun automatically advanced 2 cm out from the tip and collected the core specimen. One piece of core specimen was obtained for each puncture site in a coaxial manner as using the insulation sheath as a trocar. All procedures were performed within two hours after heparin injection without additional heparin administration in each pig.
For the control group, the core biopsy needle and the insulation sheath were removed together after biopsy. Any blood from the site was soaked up with dry gauze pads for five minutes without touching the needle tract (Fig. 2C). Bleeding amount was assessed by weighing the gauze pads before and after soaking with blood (121314). A scale (Micro Weighing Scale; CAS, Seoul, Korea) that could measure up to three-digit decimals of a gram was used. If the bleeding did not stop within five minutes, it was controlled using the Electrocauterization Unit (Force FX™ Electorsurgical Generator; Covidien, Boulder, CO, USA). After measuring the amount of blood loss, the operator cleaned the surface of the liver using dry gauze pad to avoid contamination of the results. If there was no bleeding along the biopsy tract for 2 minutes, the operator performed the next biopsy at another site (Fig. 2D).
For the experimental group, after biopsy needle removed, an electrode was inserted into the insulation sheath and the active tip length was 2 cm when combined with the insulation sheath. We used a 200-W generator (VIVA RF System; STARmed). The needle tract was ablated with 40 W of power, based on findings from the preliminary study. The operator monitored the temperature displayed in the generator, and retracting the electrode when it reached 70℃. Thus, the temperature was maintained within 70–80℃ by iterative pulling and staying of the electrode in combination with the insulation sheath. Tract ablation took about 10 seconds. Assessing blood loss in the experimental group was same, as described for the control group.
Bleeding control and maintenance of hemostasis occurred most effectively with the following ablation parameters: 2-cm active tip length, 40 W of power, and an electrode-tip temperature range of 70–80℃ during tract ablation. These ablation parameters were then used for the experimental phase.
The RF electrode with a 1-cm active tip was not effective for maintaining the temperature within 70–80℃ during ablation period. The temperature of the 1-cm electrode tip dropped shortly when extracting the electrode and reached 100℃ soon after the electrode was placed. The overheating of the electrode resulted in carbonization of the electrode surface and adhesion to the surrounding tissues. To avoid overheating of the electrode, the operator attempted to withdraw it in haste; however, this did not allow sufficient time to acquire effective coagulation. On the other hand, keeping the temperature within the stable range of 70–80℃ was easier with the 2-cm active tip than the 1-cm active tip.
Table 1 represented the results from laboratory tests before and after intravenous administration of heparin. After heparinization, two pigs (No. 3 and 4) acquired a bleeding tendency, which prolonged the aPTT level. All other results from the laboratory tests were within the normal range.
Blood loss for each liver biopsy with either 18- or 16-gauge needles in the tract ablation and non-tract ablation groups within each non-heparinized and heparinized pig were summarized in Table 2. In the two non-heparinized pigs, bleeding amounts in the experimental group were smaller than those in the control group, although the difference was only significant for the pig that received the 18-gauge biopsy needle (p < 0.001). In two heparinized pigs, the bleeding amounts in the experimental group were significantly smaller than those in the control group (p < 0.001). In the heparinized pigs, the mean amount of bleeding in the experimental group was 3.5% of the controls using an 18-gauge needle (experimental group vs. control group = 0.066 g vs. 1.862 g) and 13.5% of the control group using a 16-gauge needle (0.286 g vs. 2.120 g).
The present study was based on our earlier finding that hemostatic effects of the RF electrode path minimizes the possibility of bleeding, even in patients with bleeding tendencies or artificially infused ascites during the hepatic tumor RF ablation (15). The result from the present study demonstrates hemostatic effect by thermal cauterization of the biopsy tract using RF energy, corroborating previous reports (11121314), as well as in cases with high bleeding risk, including anti-coagulated status and use of a large bore (16-gauge) biopsy needle. Pritchard et al. (13) found that RF ablation after liver biopsy reduced bleeding by 63% in the non-heparinized pig model. In another study published by Laeseke et al. (12), mean blood loss after biopsy tract ablation was 0.1% in non-heparinized and 60% in heparinized pigs after non-tract ablation. In this study, mean blood loss after tract ablation, even in the heparinized pigs after non-tract ablation was 3.5% and 13.5%, using either the 18- or 16-gauge biopsy needle (17- and 15-gauge insulation sheath), respectively.
Many studies have been revealed effective hemostasis with embolization along the biopsy tract. Allison and Adam (5) reported their experience using steel coil under fluoroscopic guidance. Animal experimental studies using fibrin sealant for hemostasis also showed no significant bleeding complications (58). Several studies have explored embolization of gelatin particles and sponges under US and/or fluoroscopic guidance (7910). However, Choi et al. (14) reported that RF ablation was the most useful bleeding reduction method in terms of the amount of bleeding and procedure time, among embolization using an absorbable gelatin sponge, and a Histoacryl-Lipiodol mixture plugging after splenic core needle biopsy. While their experiment was conducted on splenic biopsy in a dog model, the result was not expected to differ from the current study.
A new biopsy tract ablator is feasible for even percutaneous clinical application. After acquiring the sufficient core specimens in a coaxial manner, one can change the biopsy needle with the RF electrode through the insulating sheath, allowing thermal coagulation of the biopsy tract by slowly retracting the assembled insulating sheath with the RF electrode. Previous studies (161718) demonstrated that the local temperature for optimal ablation was maintained between 60 and 100℃, with nearly instantaneous tissue coagulation necrosis. If the tissue temperature increases over these values during the procedure, tissue carbonization and adhesion are possible around the electrode. In addition, it is difficult to retract the electrode smoothly. Unlike usual RF device for tumor ablation, this device does not contain a cooling system in the electrode. Thus, the operator has to monitor the temperature of electrode tip for adequate hemostasis.
To date, the molecular-biological information in the treatment of hepatic malignancy has not impacted treatment decisions. However, in the era of molecular and personalized medicine, clinical needs for tissue acquisition before any treatment will be increased for genetic analysis to easily risk-stratify patients, identify dominant oncogenic pathways, and institute-targeted and curative therapies in the near future (1920). Thus, the amount of tissues needed for genetic analysis increases and requires more core tissues from biopsy. Ablation with this new biopsy tract ablator results in adequate hemostasis, as well as avoidance of unnecessary hepatic capsule puncture, thus saving time from needless US-guidance from the skin via co-axial technique during the procedure. Other advantages from the co-axial technique of hepatic core biopsy and the thermal ablation of needle tract have been reported. Two recent studies (2122) have shown that they may be helpful to prevent the needle tract tumor implantation. However, this was not addressed in the current study.
This study had several limitations. First, we obtained a single core specimen from one puncture. While, in actual clinical settings, ≥ 3 biopsies are obtained at an anatomical site. Greater number of biopsy cores would result in increased difference in the bleeding amount between the groups. Second, all procedures performed in this study were conducted in an intraoperative setting to accurately compare the efficacy of the RF biopsy tract coagulator in maintaining hemostasis. Thus, we could not explore the influence on hemostasis of overlaying tissues (i.e., skin, muscle, and fat) or position changes that tend to compress the biopsy site, which may play an important role in maintaining hemostasis in clinical settings. In addition, comparison of delayed bleeding or imaging studies between the two experimental groups was not available. Finally, only one pig was allocated to each experimental setting, which limits the ability to distinguish between statistical trends and individual variation.
In conclusion, the results from the in vivo porcine model experiments indicated that the new RF device for coagulating the biopsy tract may be effective at controlling post-biopsy bleeding, even in the anti-coagulated state and when using a larger (16-gauge) core biopsy needle.
Figures and Tables
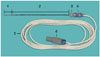 | Fig. 1Newly developed thermocouple-monitored radiofrequency biopsy tract ablator.Insulation sheath with electrode in place: device is composed of 1) 2 cm-long active electrode tip, 2) insulation sheath covered by polyester, 3) sheath handle, 4) electrode handle, 5) multi cable, and 6) connector.
|
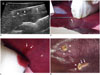 | Fig. 2US-guided biopsy procedure and assessment of blood loss from biopsy site.
A. US image during tract ablation procedure after core biopsy, combination of 2-cm active electrode tip (arrowheads, “1” in Figure 1) and insulation sheath (arrow, “2” in Figure 1) retracted together while monitoring temperature of electrode tip. B. Insulation sheath with engaged automated biopsy gun in place. 0.5-cm biopsy needle tip (arrow) protrudes from end of insulation sheath. US-guided liver biopsy was performed in coaxial manner through insulation sheath. C. Any blood from site (arrows) was soaked with dry gauze pads for five minutes without touching needle tract and was estimated by reweighing gauze pads. D. Two biopsy sites after measuring amount of blood loss and electrocauterization. Lesion at left upper was control biopsy site and lesion in right lower was experimental biopsy site. Arrowheads and arrows demonstrate electrocauterized points and RF electrode path with burn, respectively. RF = radiofrequency, US = ultrasound
|
Table 1
Results of Laboratory Tests on Coagulopathy before and/or after Heparinization

Table 2
Comparison of Mean Blood Loss between Control (Non-Tract Ablation) and Experimental (Tract Ablation) Group in Each Pig

Acknowledgments
All applicable institutional and/or national guidelines for the care and use of animal were followed. This study was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of the Samsung Biomedical Research Institute (SBRI). SBRI is an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International) and an accredited facility that abides by the Institute of Laboratory Animal Resources (ILAR) guide.
References
1. Korean Liver Cancer Study Group (KLCSG). National Cancer Center, Korea (NCC). 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015; 16:465–552.
2. Ma X, Arellano RS, Gervais DA, Hahn PF, Mueller PR, Sahani DV. Success of image-guided biopsy for small (≤ 3 cm) focal liver lesions in cirrhotic and noncirrhotic individuals. J Vasc Interv Radiol. 2010; 21:1539–1547. quiz 1547.
3. Sporea I, Gherhardt D, Popescu A, Sirli R, Cornianu M, Herman D, et al. Does the size of the needle influence the number of portal tracts obtained through percutaneous liver biopsy? Ann Hepatol. 2012; 11:691–695.
4. McAfee JH, Keeffe EB, Lee RG, Rösch J. Transjugular liver biopsy. Hepatology. 1992; 15:726–732.
5. Allison DJ, Adam A. Percutaneous liver biopsy and track embolization with steel coils. Radiology. 1988; 169:261–263.
6. Falstrom JK, Moore MM, Caldwell SH, Matsumoto AH, Abbott RD, Spotnitz WD. Use of fibrin sealant to reduce bleeding after needle liver biopsy in an anticoagulated canine model: work in progress. J Vasc Interv Radiol. 1999; 10:457–462.
7. Fandrich CA, Davies RP, Hall PM. Small gauge gelfoam plug liver biopsy in high risk patients: safety and diagnostic value. Australas Radiol. 1996; 40:230–234.
8. Paulson EK, Stephenson GR, Neal MC, Rossin V, Lawson JH. Use of fibrin sealant as a hemostatic agent after liver biopsy in swine. J Vasc Interv Radiol. 2000; 11:905–911.
9. Smith TP, McDermott VG, Ayoub DM, Suhocki PV, Stackhouse DJ. Percutaneous transhepatic liver biopsy with tract embolization. Radiology. 1996; 198:769–774.
10. Zins M, Vilgrain V, Gayno S, Rolland Y, Arrivé L, Denninger MH, et al. US-guided percutaneous liver biopsy with plugging of the needle track: a prospective study in 72 high-risk patients. Radiology. 1992; 184:841–843.
11. Bruners P, Penzkofer T, Isfort P, Pfeffer J, Schmitz-Rode T, Günther RW, et al. A trucut biopsy needle for bipolar radiofrequency ablation of needle tract: a proof-of-concept experiment. Eur Radiol. 2010; 20:2000–2004.
12. Laeseke PF, Winter TC 3rd, Davis CL, Stevens KR, Johnson CD, Fronczak FJ, et al. Postbiopsy bleeding in a porcine model: reduction with radio-frequency ablation--preliminary results. Radiology. 2003; 227:493–499.
13. Pritchard WF, Wray-Cahen D, Karanian JW, Hilbert S, Wood BJ. Radiofrequency cauterization with biopsy introducer needle. J Vasc Interv Radiol. 2004; 15(2 Pt 1):183–187.
14. Choi SH, Lee JM, Lee KH, Kim SH, Lee JY, Han JK, et al. Postbiopsy splenic bleeding in a dog model: comparison of cauterization, embolization, and plugging of the needle tract. AJR Am J Roentgenol. 2005; 185:878–884.
15. Kang TW, Lim HK, Lee MW, Kim YS, Choi D, Rhim H. First-line radiofrequency ablation with or without artificial ascites for hepatocellular carcinomas in a subcapsular location: local control rate and risk of peritoneal seeding at long-term follow-up. Clin Radiol. 2013; 68:e641–e651.
16. Nahum Goldberg S, Dupuy DE. Image-guided radiofrequency tumor ablation: challenges and opportunities--part I. J Vasc Interv Radiol. 2001; 12:1021–1032.
17. McGhana JP, Dodd GD 3rd. Radiofrequency ablation of the liver: current status. AJR Am J Roentgenol. 2001; 176:3–16.
18. Zervas NT, Kuwayama A. Pathological characteristics of experimental thermal lesions. Comparison of induction heating and radiofrequency electrocoagulation. J Neurosurg. 1972; 37:418–422.
19. Chan SL, Wong AM, Lee K, Wong N, Chan AK. Personalized therapy for hepatocellular carcinoma: where are we now? Cancer Treat Rev. 2016; 45:77–86.
20. Zhang B, Finn RS. Personalized clinical trials in hepatocellular carcinoma based on biomarker selection. Liver Cancer. 2016; 5:221–232.
21. Chang S, Kim SH, Lim HK, Kim SH, Lee WJ, Choi D, et al. Needle tract implantation after percutaneous interventional procedures in hepatocellular carcinomas: lessons learned from a 10-year experience. Korean J Radiol. 2008; 9:268–274.
22. Kim JW, Shin SS, Heo SH, Hong JH, Lim HS, Seon HJ, et al. Ultrasound-guided percutaneous radiofrequency ablation of liver tumors: how we do it safely and completely. Korean J Radiol. 2015; 16:1226–1239.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download