Abstract
Reconstruction of a ruptured anterior cruciate ligament (ACL) is a well-established procedure for repair of ACL injury. Despite improvement of surgical and rehabilitation techniques over the past decades, up to 25% of patients still fail to regain satisfactory function after an ACL reconstruction. With development of CT imaging techniques for reducing metal artifacts, multi-planar reconstruction, and three-dimensional reconstruction, early post-operative imaging is increasingly being used to provide immediate feedback to surgeons regarding tunnel positioning, fixation, and device placement. Early post-operative radiography and CT imaging are easy to perform and serve as the baseline examinations for future reference.
The anterior cruciate ligament (ACL) is the most frequently reported injured knee ligament that requires surgical reconstruction. ACL injuries most commonly occur in athletes playing multidirectional sports (12). Imaging is frequently used to evaluate complications in patients with postoperative symptoms. Hence, radiologists should be familiar with the normal postoperative appearance and complications that can be diagnosed with imaging. The purpose of this article is to review brief surgical techniques, surgical navigation terminology, and anatomic positions of tunnels and fixation devices. We also review procedure-related radiographic and CT imaging findings after ACL reconstruction with examples of measurements and abnormal findings in the post-operative phase.
The ACL consists of two major functional bundles: the anteromedial (AM) bundle and the posterolateral (PL) bundle. The bundles are named according to their insertion on the tibia. Both contribute substantially to the anterior and rotational stability of the knee. The AM bundle is inserted more anteromedially on the tibia and originates more proximally on the femur than the PL bundle (345). The two bundles run parallel on knee extension during which the AM bundle loosens and the PL bundle tightens; and cross on knee flexion, when the AM bundle tightens and the PL bundle loosens (Fig. 1) (678). The PL bundle also tightens during internal and external knee rotation (6).
Single-bundle, double-bundle, and selective single-bundle augmentation techniques are widely practiced in ACL reconstruction (Fig. 2) (9). A single-bundle reconstruction is performed by producing one single femoral tunnel and one single tibial tunnel, with focus on reproducing the AM bundle. The selective single-bundle augmentation reconstruction is focused on AM or PL bundle repair with preservation of the remaining intact bundle, while the double-bundle reconstruction uses two separate grafts to replace the positioning of both the AM and PL bundles (9). An anatomic double-bundle, four-tunnel reconstruction using the double-bundle technique has the potential to restore the biomechanics of the knee better than the classic single-bundle, two-tunnel technique (Fig. 3) (10). However, similar long-term results for both techniques are reported in a previous study (11). The double-bundle and single-bundle techniques, aim to place the tunnels in the anatomically correct insertion sites of the native ACL on the femur and tibia, respectively (1213). A recent systematic review (14) comparing single- and double-bundle ACL reconstructions shows that double-bundle reconstruction has fewer re-ruptures and less anteroposterior and rotatory laxity, while complications of double-bundle and selective single-bundle augmentation ACL reconstructions such as graft disruption, graft impingement, arthrofibrosis, hardware failure, and dislodgement are similar to those of single-bundle reconstructions (4).
Since surgery is performed with the knee in flexion, surgical terminology differs from anatomical terminology (Fig. 4). The following are the most commonly used terms for navigating in the femoral intercondylar notch: shallow or deep and high or low and should avoid confusion with anatomical position.
Malpositioning of the bone tunnels is considered as one of the most common technical errors in ACL reconstruction (15). It is estimated that up to 80% of technical failures are based on improper tunnel placement (16). When used, radiographic and three dimensional CT measurements show reliable correlation with anatomic dissection measurements of ACL insertion sites (17).
In anatomic single-bundle ACL reconstruction, the femoral tunnel is placed at the site of insertion of the native ACL. Correct tunnel positioning is essential for an optimum clinical outcome in all these techniques (18). Blumensaat's line and "Bernard and Hertel grid" are commonly adopted radiographic markers to determine the location of the tunnels in the distal femoral shaft (19). In this grid-based technique, the optimal placement for deep-shallow direction has a ratio of 24 to 27%. For the optimal placement for the high-low direction, a ratio of 28 to 34% is proposed (Fig. 5A) (2021). A superficial placement is the most frequent malposition of the femoral tunnel (22). The angle measured between a line drawn along the femur diaphysis and the femoral tunnel angle must be approximately 39°. Angles of approximately ≤ 17° are associated with rotational instability (Fig. 5B) (2324).
The single-bundle graft is required to provide both anterior-posterior and rotational (pivotal) stability. The optimal placement is within the anatomic footprint (Fig. 6A). On coronal plane, its center should enter the intercondylar notch 2–3 mm posterior to the normal distal ACL insertion on the tibial plateau (Fig. 6B) (25).
The Amis and Jakob line is one of the most commonly used methods to evaluate the anterior-posterior direction of the tibial tunnel (Fig. 6C), which passes through the widest part of the posterior corner of medial tibial plateau, parallel to the medial joint line (20). The measurement originally performed on a mid-sagittal MR image is reported at around 43%. Normal values range between 27 and 60% (2026). The entire opening of the tibial tunnel must be located dorsally to the line drawn along the Blumensaat's line (Fig. 6C) (27). When the femoral tunnel is drilled through the tibial tunnel, it is recommended to drill the tibial tunnel at an angle of 65 degrees to 70 degrees in the coronal plane (Fig. 7) (27).
Recent advances in multi-detector CT technology facilitate the acquisition of isotropic data in nearly every CT examination. Multi-detector CT technology has the ability to create multi-planar reformation and volume rendering for the creation of three-dimensional images. Post-processing methods after ACL reconstruction surgery vary because of differences in available equipment and personal preferences. CT scans and three-dimensional volume rendering images are more reliable in assessing postoperative bone tunnel placement following ACL reconstruction than standard radiographs (28). We create orthogonal coronal and sagittal plane reformat images and two volume rendered images for radiological assessment of tunnel position. To access femoral tunnel location, a volume-rendered image of lateral view on the lateral femoral condyle (Fig. 5A) is reconstructed. Tibial tunnel placement is intuitively recognized on cranial view of the tibia's volume rendered image (Fig. 6A) (2428). Although bone tunnel widening is usually assessed with plain radiographs, CT reconstructions aligned along the axis of the bone tunnel are helpful for follow-up in tunnel widening, especially when multiple tunnels exist e.g., after revision surgery (Fig. 8) (29).
When femoral tunnel placement is too shallow and too high, the graft is taut in flexion. If tunnel placement is too high, the graft may overstretch in extension and reduce the range of motion (Fig. 9) (24). If the tibial tunnel position is too anterior, it might result in pathological impingement of the ACL onto the notch roof, resulting in extension deficit. In contrast, if the tibial tunnel position is too posterior, it might result in persistent instability (Fig. 10) (30). Rotational instability is associated, if the tibial and femoral tunnels are too steep. Femoral tunnel angles < 17° and tibial tunnel angles > 72° are indicative of an unstable knee joint (Fig. 7B).
Hamstring grafts are fixed with a device (like a button) to suspend it at the femur and a screw (for instance bioabsorbable screw) to fix it in the tibia. The EndoButton (Acufex Microsurgical, Mansfield, MA, USA) is one of the most often used materials for fixation in recent years. Although it achieves a rapid and secure fixation, button style extra-cortical fixation device can slide into the tunnel (Fig. 11) (31). Possible interpositioning of tissue between extra-cortical devices and the cortex has no effect on the long-term outcome (Fig. 12) (32). Patellar tendon grafts are usually fixed with two interference screws, as they have bony attachments at each end. Since migration of screw is a potential complication of radiolucent "bioabsorbable" interference screws (33), radiologists should carefully examine postoperative images (Fig. 13).
Tunnel enlargement after ACL reconstruction is a well-known phenomenon that predominantly occurs during the first six months after surgery, and represents a potential problem for revision surgery (34). Early post-operative imaging is used as a baseline for future reference. Because tunnels are originally drilled with a bore, the tunnels should have parallel walls. Any change in parallel walls (into a cone shaped tunnel) should raise suspicion of tunnel widening. Tunnel widening can be defined as postoperative enlargement > 2 mm on antero-posterior or lateral radiographs (Fig. 14) (35).
Protrusion of screw tip into calf muscles can cause indentation and popliteal area pain. Intramuscular location of a screw tip may be detected in routine post-operative CT scan (Fig. 15).
Interference screws provide the most secure fixation in the immediate postoperative period, and the optimal orientation of the screw within the tunnel for maximum fixation strength is parallel to the graft. If the screws diverge or converge, fixation strength may be compromised. The divergence angle is the angle between a line drawn down the long axis of the screw and a line drawn down the long axis of the tunnel (Fig. 2A), and should not exceed 15 to 30° (36).
Any case resulting in intra-operative bone fractures can be fixated during the operation or can heal spontaneously without complication. Although, few reports describe tibial plateau fracture complicating ACL reconstruction after 7 to 18 months postoperatively and are induced by torsional trauma (37). Although aseptic wound healing problems can be managed without revision of ACL, septic arthritis after ACL reconstruction is a rare but potentially devastating complication. The correct diagnosis relies on clinical evaluation, laboratory tests, synovial fluid analysis, and bacterial culture. The infection can be successfully managed with early diagnosis and prompt treatment (38).
Given the increasing number of patients undergoing ACL reconstruction, it is imperative for radiologists to be familiar with these procedures and the associated abnormalities. In addition, adhering to recommended assessment criteria, measurements, and terminology is crucial. Early, post-operative radiographic and CT images are easy to obtain and provide good information regarding tunnel position, bony structures, and fixation devices. Imaging serves as a baseline examination for further follow-ups, helps avoid confusion, and increases the usefulness of the reports.
Figures and Tables
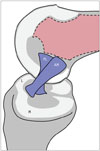 | Fig. 1Schematic drawing of ACL bundles in flexed knee.Anteromedial (AM) bundle includes fascicles attached to proximal part of femoral attachment site and to anteromedial aspect of tibial attachment. Posterolateral (PL) bundle consists of fascicles attached to femur distally and to tibia posterolaterally. If knee is extended, PL bundle is taut and has appearance of being flat and broad. On contrary, when knee is flexed, AM bundle becomes taut. ACL = anterior cruciate ligament, L = lateral, M = medial
|
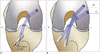 | Fig. 2Illustrations of anteroposterior view of knee showing technique for single bundle (A) and double bundle reconstruction (B) of ACL.ACL = anterior cruciate ligament, AM = anteromedial bundle, L = lateral, M = medial, PL = posterolateral bundle
|
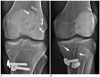 | Fig. 3Tunnel position after double-bundle ACL reconstruction reflects natural course of two ACL bundles.
A. Anteroposterior radiograph showing greater proximal and anterior course of anteromedial (AM) graft (1-o'clock), as compared to posterolateral (PL) graft (2-o'clock) on lateral femoral condyle. B. On oblique radiograph, AM bundle tunnel shows more anterior course than PL bundle tunnel. ACL = anterior cruciate ligament
|
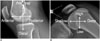 | Fig. 4Terms and directions used in anatomy and radiology (A) and terms and directions used in surgery (B).drawing of eye in (B) represents view in surgery.
|
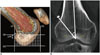 | Fig. 5Normal femoral tunnel position.
A. Use of Bernhard and Hertel grid to assess femoral tunnel placement. a = Blumensaat's line: tangent to roof of intercondylar notch, b = Parallel to Blumensaat's line and tangent to inferior border of condyle, c = Perpendicular to Blumensaat's line, at intersection of tangent line with deep border of lateral femoral condyle, d = Perpendicular to Blumensaat's line, at intersection of tangent line with shallow border of lateral femoral condyle. Dotted circle = ideal location, 27% deep-shallow and 34% high-low. B. Angle measurement of femoral tunnel to femur on coronal CT image using picture archiving and communication system software.
|
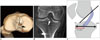 | Fig. 6Normal tibial tunnel position.
A. Tibial tunnel (white arrow) is placed at site of tibial footprint; black arrow indicates tibial spine. B. Tibial tunnel (arrow) enters intercondylar notch, in between tibial spines on coronal CT. C. Schematic drawing of lateral view of knee shows intercondylar portion of graft is oriented taut and parallel to or steeper than Blumensaat's line (black line), and entire tunnel is positioned posterior to line extended along Blumensaat's line. Anterior-posterior position of tibial tunnel as ratio is also depicted. Most anterior vertical line indicates 0% and most posterior vertical line, 100% on Amis and Jakob line (black double arrows), respectively. Position of center of ACL tibial insertion (red double arrows) should lie between 27 and 60% along Amis and Jakob line. ACL = anterior cruciate ligament
|
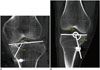 | Fig. 7Angular measurement of tibial tunnel.
A. Angle of tibial tunnel with transtibial technique. Angle should not exceed 72°. B. Example of too steep tibial tunnel. Tibial tunnel angle of ≥ 72° is associated with greater loss of flexion and anterior laxity. In this case, angle of tibial tunnel was 75°.
|
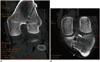 | Fig. 8Normal femoral and tibial tunnels on multiplanar reformat CT images.Oblique coronal multiplanar reformat images aligned along axes of femoral (A) and tibial (B) tunnels clearly demonstrate entire course and width (double arrows) of both tunnels with parallel walls.
|
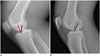 | Fig. 9Schematic drawing of kinematics of graft on flexion (A) and extension (B).Red line demonstrates taut graft in too shallow and too high placed tunnels. If tunnel placement is too high (blue line), graft will be over stretched in extension and may reduce range of motion. Optimal graft placement is indicated with black lines.
|
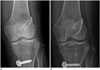
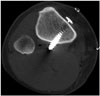
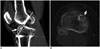 | Fig. 10Example of tibial tunnel positioned too anteriorly.Sagittal CT image reveals tibial tunnel is drilled anterior to Blumensaat's line (white line). Greater anterior placement of tibial tunnel will cause impingement of graft during extension. Note fracture (arrows) in roof of tibial tunnel on sagittal (A) and axial (B) images.
|
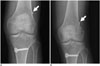 | Fig. 11Migration of button style extra-cortical fixation device.Images obtained immediately (A) and 6 months after surgery (B) show mild sliding of EndoButton fixation device into femoral tunnel (arrows).
|
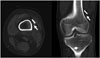 | Fig. 12Gap between fixation device and bone cortex.On axial and coronal post-operative CT scans, gap is seen (arrows) between cortex and fixation device, caused by tissue interposition
|
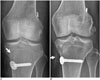 | Fig. 13Migration of bioabsorbable interference screws.Compared to that seen on radiograph obtained immediately after surgery (A), tibial fixation screw (arrow) can be seen protruding into anterior knee on radiograph obtained on 6-month follow-up (B). It is easily overlooked, as bioabsorbable screws are radiolucent.
|
References
1. Gianotti SM, Marshall SW, Hume PA, Bunt L. Incidence of anterior cruciate ligament injury and other knee ligament injuries: a national population-based study. J Sci Med Sport. 2009; 12:622–627.
2. Janssen KW, Orchard JW, Driscoll TR, van Mechelen W. High incidence and costs for anterior cruciate ligament reconstructions performed in Australia from 2003-2004 to 2007-2008: time for an anterior cruciate ligament register by Scandinavian model? Scand J Med Sci Sports. 2012; 22:495–501.
3. Adriaensen ME, Hogan B, Al-Bulushi HI, Kavanagh EC. Double-bundle depiction of the anterior cruciate ligament at 3 Tesla. Skeletal Radiol. 2012; 41:831–834.
4. Casagranda BU, Maxwell NJ, Kavanagh EC, Towers JD, Shen W, Fu FH. Normal appearance and complications of double-bundle and selective-bundle anterior cruciate ligament reconstructions using optimal MRI techniques. AJR Am J Roentgenol. 2009; 192:1407–1415.
5. Ng AW, Griffith JF, Hung EH, Law KY, Yung PS. MRI diagnosis of ACL bundle tears: value of oblique axial imaging. Skeletal Radiol. 2013; 42:209–217.
6. Torabi M, Fu F, Luo J, Costello J. Clinical relevance and imaging features of isolated single bundle anterior cruciate tear and single bundle augmentation. Clin Imaging. 2013; 37:830–835.
7. Gabriel MT, Wong EK, Woo SL, Yagi M, Debski RE. Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. J Orthop Res. 2004; 22:85–89.
8. Sakane M, Fox RJ, Woo SL, Livesay GA, Li G, Fu FH. In situ forces in the anterior cruciate ligament and its bundles in response to anterior tibial loads. J Orthop Res. 1997; 15:285–293.
9. Ma Y, Deie M, Iwaki D, Asaeda M, Fujita N, Adachi N, et al. Balance ability and proprioception after single-bundle, single-bundle augmentation, and double-bundle ACL reconstruction. ScientificWorldJournal. 2014; 2014:342012.
10. Bencardino JT, Beltran J, Feldman MI, Rose DJ. MR imaging of complications of anterior cruciate ligament graft reconstruction. Radiographics. 2009; 29:2115–2126.
11. Tiamklang T, Sumanont S, Foocharoen T, Laopaiboon M. Double-bundle versus single-bundle reconstruction for anterior cruciate ligament rupture in adults. Cochrane Database Syst Rev. 2012; 11:CD008413.
12. Trojani C, Beaufils P, Burdin G, Bussière C, Chassaing V, Djian P, et al. Revision ACL reconstruction: influence of a lateral tenodesis. Knee Surg Sports Traumatol Arthrosc. 2012; 20:1565–1570.
13. Muneta T, Sekiya I, Yagishita K, Ogiuchi T, Yamamoto H, Shinomiya K. Two-bundle reconstruction of the anterior cruciate ligament using semitendinosus tendon with endobuttons: operative technique and preliminary results. Arthroscopy. 1999; 15:618–624.
14. Björnsson H, Desai N, Musahl V, Alentorn-Geli E, Bhandari M, Fu F, et al. Is double-bundle anterior cruciate ligament reconstruction superior to single-bundle? A comprehensive systematic review. Knee Surg Sports Traumatol Arthrosc. 2015; 23:696–739.
15. Denti M, Lo Vetere D, Bait C, Schönhuber H, Melegati G, Volpi P. Revision anterior cruciate ligament reconstruction: causes of failure, surgical technique, and clinical results. Am J Sports Med. 2008; 36:1896–1902.
16. Haasper C, Kopf S, Lorenz S, Middleton KK, Tashman S, Fu FH. Influence of tibial rotation on tibial tunnel position measurements using lateral fluoroscopy in anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2015; 23:649–654.
17. Lee JK, Lee S, Seong SC, Lee MC. Anatomy of the anterior cruciate ligament insertion sites: comparison of plain radiography and three-dimensional computed tomographic imaging to anatomic dissection. Knee Surg Sports Traumatol Arthrosc. 2015; 23:2297–2305.
18. Bedi A, Maak T, Musahl V, O'Loughlin P, Choi D, Citak M, et al. Effect of tunnel position and graft size in single-bundle anterior cruciate ligament reconstruction: an evaluation of time-zero knee stability. Arthroscopy. 2011; 27:1543–1551.
19. Bernard M, Hertel P, Hornung H, Cierpinski T. Femoral insertion of the ACL. Radiographic quadrant method. Am J Knee Surg. 1997; 10:14–21. discussion 21-22.
20. Amis AA, Jakob RP. Anterior cruciate ligament graft positioning, tensioning and twisting. Knee Surg Sports Traumatol Arthrosc. 1998; 6:Suppl 1. S2–S12.
21. Bird JH, Carmont MR, Dhillon M, Smith N, Brown C, Thompson P, et al. Validation of a new technique to determine midbundle femoral tunnel position in anterior cruciate ligament reconstruction using 3-dimensional computed tomography analysis. Arthroscopy. 2011; 27:1259–1267.
22. Tscholl PM, Biedert RM, Gal I. Radiological evaluation for conflict of the femoral tunnel entrance area prior to anterior cruciate ligament revision surgery. Int Orthop. 2014; 38:607–615.
23. Illingworth KD, Hensler D, Working ZM, Macalena JA, Tashman S, Fu FH. A simple evaluation of anterior cruciate ligament femoral tunnel position: the inclination angle and femoral tunnel angle. Am J Sports Med. 2011; 39:2611–2618.
24. Parkar AP, Adriaensen ME, Strand T, Inderhaug E, Harlem T, Solheim E. How to read post-operative radiographs and CT scans after single-bundle anterior cruciate ligament reconstruction. Skeletal Radiol. 2013; 42:1489–1500.
25. Howell SM, Gittins ME, Gottlieb JE, Traina SM, Zoellner TM. The relationship between the angle of the tibial tunnel in the coronal plane and loss of flexion and anterior laxity after anterior cruciate ligament reconstruction. Am J Sports Med. 2001; 29:567–574.
26. Stäubli HU, Rauschning W. Tibial attachment area of the anterior cruciate ligament in the extended knee position. Anatomy and cryosections in vitro complemented by magnetic resonance arthrography in vivo. Knee Surg Sports Traumatol Arthrosc. 1994; 2:138–146.
27. Howell SM, Hull ML. Checkpoints for judging tunnel and anterior cruciate ligament graft placement. J Knee Surg. 2009; 22:161–170.
28. Meuffels DE, Potters JW, Koning AH, Brown CH Jr, Verhaar JA, Reijman M. Visualization of postoperative anterior cruciate ligament reconstruction bone tunnels: reliability of standard radiographs, CT scans, and 3D virtual reality images. Acta Orthop. 2011; 82:699–703.
29. Yoon SJ, Yoon YC, Bae SY, Wang JH. Bone tunnel diameter measured with CT after anterior cruciate ligament reconstruction using double-bundle auto-hamstring tendons: clinical implications. Korean J Radiol. 2015; 16:1313–1318.
30. Kondo E, Yasuda K, Ichiyama H, Azuma C, Tohyama H. Radiologic evaluation of femoral and tibial tunnels created with the transtibial tunnel technique for anatomic double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2007; 23:869–876.
31. Yanmiş I, Tunay S, Oğuz E, Yildiz C, Ozkan H, Kirdemir V. Dropping of an EndoButton into the knee joint 2 years after anterior cruciate ligament repair using proximal fixation methods. Arthroscopy. 2004; 20:641–643.
32. Mae T, Kuroda S, Matsumoto N, Yoneda M, Nakata K, Yoshikawa H, et al. Migration of EndoButton after anatomic double-bundle anterior cruciate ligament reconstruction. Arthroscopy. 2011; 27:1528–1535.
33. Pereira H, Correlo VM, Silva-Correia J, Oliveira JM, Reis RL, Espregueira-Mendes J. Migration of "bioabsorbable" screws in ACL repair. How much do we know? A systematic review. Knee Surg Sports Traumatol Arthrosc. 2013; 21:986–994.
34. Fauno P, Kaalund S. Tunnel widening after hamstring anterior cruciate ligament reconstruction is influenced by the type of graft fixation used: a prospective randomized study. Arthroscopy. 2005; 21:1337–1341.
35. Choi NH, Lee JH, Son KM, Victoroff BN. Tibial tunnel widening after anterior cruciate ligament reconstructions with hamstring tendons using Rigidfix femoral fixation and Intrafix tibial fixation. Knee Surg Sports Traumatol Arthrosc. 2010; 18:92–97.
36. Fineberg MS, Zarins B, Sherman OH. Practical considerations in anterior cruciate ligament replacement surgery. Arthroscopy. 2000; 16:715–724.
37. Mithöfer K, Gill TJ, Vrahas MS. Tibial plateau fracture following anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2004; 12:325–328.
38. Wang C, Ao Y, Wang J, Hu Y, Cui G, Yu J. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: a retrospective analysis of incidence, presentation, treatment, and cause. Arthroscopy. 2009; 25:243–249.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download