Abstract
Objective
To retrospectively evaluate the short-term outcomes and safety of computed tomography (CT)-guided percutaneous microwave ablation (MWA) of solitary adrenal metastasis from lung cancer.
Materials and Methods
From May 2010 to April 2014, 31 patients with unilateral adrenal metastasis from lung cancer who were treated with CT-guided percutaneous MWA were enrolled. This study was conducted with approval from local Institutional Review Board. Clinical outcomes and complications of MWA were assessed.
Results
Their tumors ranged from 1.5 to 5.4 cm in diameter. After a median follow-up period of 11.1 months, primary efficacy rate was 90.3% (28/31). Local tumor progression was detected in 7 (22.6%) of 31 cases. Their median overall survival time was 12 months. The 1-year overall survival rate was 44.3%. Median local tumor progression-free survival time was 9 months. Local tumor progression-free survival rate was 77.4%. Of 36 MWA sessions, two (5.6%) had major complications (hypertensive crisis).
Lung cancer is the most common primary tumor that metastasizes to adrenal glands (1). Several investigators have emphasized that adrenalectomy could increase the survival rates of patients with isolated metastases to adrenal glands (23). However, due to comorbid disease, age, previous surgery, and the existence of extra-adrenal tumors, few patients are surgical candidates. Presently, an increasing number of minimally invasive techniques are available for treating adrenal tumors, including arterial embolization, local chemical ablation, and local thermal ablation (456).
Microwave ablation (MWA) is one of the newest applied thermal ablation method. Its use is gradually increasing because it induces little trauma with high thermal efficiency and good repeatability for many solid malignancies, such as lung, liver, bone, kidney, and other solid tumors (7891011). However, using MWA in adrenal metastasis from lung cancer has only been reported in a few cases. The purpose of this study was to retrospectively evaluate the short-term outcomes including survival outcomes and safety of thermal ablation using MWA guided by computed tomography (CT) for isolated adrenal metastasis from lung cancer.
This retrospective study was approved by four hospitals: Shandong Provincial Hospital Affiliated with Shandong University, Sun Yat-sen University Cancer Center, Teng Zhou Central People's Hospital Affiliated with Jining Medical College, and Jinan Military General Hospital of the Chinese People's Liberation Army. From May 2010 to June 2014, 94 patients were histologically verified with adrenal metastases from lung cancer. A total of 31 patients met the following inclusion criteria: 1) the patient had unilateral adrenal metastases from lung cancer that were metachronous; 2) the patient was medically inoperable due to renal or heart dysfunction, other comorbid medical conditions (such as severe diabetes), or advanced stage; 3) the patient refused surgery; and 4) the patient did not undergo local radiotherapy without being affected by chemotherapy or tyrosine kinase inhibitors. The exclusion criteria were: 1) uncontrolled infection around the lesion or systemic infection; 2) coagulation disorder, especially in patients with platelet count of < 50 × 109/L who had received continuous anti-coagulation therapy; 3) unfit for MWA due to severely deficient hepatic, renal, or cardiopulmonary function; or 4) an Eastern Cooperative Oncology Group performance status of 0–2 (12). Patients were informed in detail of the risks and benefits associated with MWA treatment and provided written informed consent for the procedure. Ethical approval to conduct this study was obtained by the Institutional Review Boards of the participating hospitals.
A CT-guided biopsy was performed by an ablation operator for all lesions before MWA to determine tumor pathology. Pathological diagnosis was evaluated by the pathologist of the participating hospitals.
Local anesthetic and preemptive analgesia were used during MWA procedure (13). Patients abstained from solid food for 12 hours prior to the procedure. For preemptive analgesia, morphine (10 mg, subcutaneous), diazepam (10 mg, intramuscular), and flurbiprofen axetil (50 mg, intravenous) were injected before the procedure. An intravenous injection of flurbiprofen axetil (50 mg) was also administered after the procedure. Local anesthetic was applied at the selected puncture points using 2% lidocaine.
Treatment and follow-up of all patients were performed under multislice CT guidance (Lightspeed16; GE Healthcare, Waukesha, WI, USA). The adrenal gland of the patient in lateral, supine, or prone position was scanned using a slice thickness of approximately 3–5 mm. After adrenal gland CT scanning, the appropriate scanned layers were selected to confirm puncture angles and depths.
A commercially available KY-2450B MWA system (Nanjing Kang You Microwave Research Institute, Nanjing, China: SFDA certified No.: [2011] 3250282) or an ECO-2450B MWA system (ECO Microwave Electronic Institute, Nanjing, China. Registration standard: YZB/country 1475–2013. China: SFDA certified No.: [III] 20112251456) was used for MWA. In general, the ablation power selected was 60–70 W and the ablative duration ranged from 4 to 8 minutes as decribed previously (1415). For tumors larger than 3.5 cm, ablation was performed with 2 antennas (14151617). Immediately after the MWA procedure, CT scanning was performed again to evaluate the tumor size, tumor morphology, and the status of adjacent organs. In addition, any complications such as bleeding were recorded.
During the procedure, the patient's heart rate, blood pressure (BP), electrocardiogram, and peripheral blood oxygen saturation level were carefully monitored. BP monitoring was performed every 5 minutes initially and every 3 minutes after the start of the ablation using a BP cuff. Vital signs, clinical symptoms, and urine volumes were closely observed during post-MWA hospital stay.
To evaluate the efficacy of treatment, follow-up CT was performed for each patient at one month after ablation. According to Image-Guided Tumor Ablation: Standardization of Terminology and Reporting Criteria (2005 and 2014) (818), primary or secondary efficacy rate of MWA was assessed to determine local treatment efficacy. The lesion observed at one month after ablation was used as the baseline to determine local efficacy. Primary efficacy rate was defined by lesion disappearance, fibrotic progression or scar, solid nodule involution or no change, and a lack of contrast enhancement signs on CT scan following the initial ablation. Secondary efficacy rate included tumors that underwent successful repeat ablation following the identification of local tumor progression (8). Residual unablated tumor was defined when the initial follow-up CT demonstrated a focal area of definite enhancement (i.e., attenuation difference of 20 Hounsfield units or greater between pre- and post-contrast CT images) within the ablation zone. After the initial CT evaluation, CT was performed every three months. Local tumor progression was defined as the appearance of tumor foci at the edge of the ablation zone after at least one contrast-enhanced follow-up study documenting adequate ablation and an absence of viable tissue in the target tumor and surrounding ablation margin according to imaging criteria (8). Local efficacy of MWA was collectively assessed by a medical oncologist and two imaging doctors. The median follow-up duration post-ablation was 11.1 months (range, 4–32 months).
The assessment of complications and side effects was also based on Image-Guided Tumor Ablation: Standardization of Terminology and Reporting Criteria (818). Both major and minor complications were assessed. Major complications that occurred in the process of ablation or post-treatment were promptly treated. If untreated, these clinical symptoms might be life threatening and lead to dysfunction and prolonged hospital stay. Minor complications are self-limited. They lack substantial morbidity. They only require a short hospitalization period. Minor complications include pain, post-ablation syndrome, and asymptomatic bleeding or effusion that can only be seen on CT images.
Data were analyzed using IBM SPSS statistics software (version 22, Armonk, NY, USA). Chi-squared test was used to compare local recurrence rate and survival in two groups of patients (those with a tumor size larger than 3.5 cm and those with a tumor size smaller than 3.5 cm). Overall survival and local tumor progression-free survival were estimated using Kaplan-Meier method. All tests were two-sided. Statistical significance was considered when p value was less than 0.05.
The average age of the 31 patients was 64.9 years (range, 45–82 years), including 18 males and 13 females. Of the 31 adrenal metastases treated with MWA, 13 were located in the right adrenal gland and 18 were located in the left adrenal gland. The average (mean ± SD) tumor diameter was 3.46 ± 1.08 cm (range, 1.5–5.4 cm). Patient and tumor characteristics are summarized in Table 1.
The range of hospital stay was 2–3 days. Successful ablation was achieved for all patients (technical success rate of 100%). All 31 patients underwent CT scan immediately after MWA. The 31 lesions displayed lower densities and cavities of various sizes in the ablation area. Follow-up CT scan at 1 month after MWA revealed primary efficacy rate of 90.3% (28 of the 31 lesions). Three lesions (patient No. 2, 10, and 24) had residual unablated tumor. For tumors ≤ 3.5 cm, the primary efficacy rate was 100% (16/16) (Figs. 1, 2, 3).
During the follow-up period, local tumor progression was detected in 7 (22.6%) of 31 cases. Patient No. 17 experienced local progression at 3 months after MWA. Patients No. 7 and No. 27 experienced local progression at 4 month and 5 months after MWA, respectively. MWA was repeated for patient No. 7. The tumor did not progress during the remainder of the follow-up period. Two patients (No. 2 and No. 13) experienced progression after 6 months post-MWA. Patient No. 2 experienced residual unablated tumor after the initial MWA but achieved secondary efficacy after the second MWA. However, this patient's lesion still progressed. Patients No. 28 and No. 19 experienced local progression after 8 and 9 months post-MWA, respectively. The secondary efficacy rate was 66.7% (MWA was repeated in the 3 patients following local progression, 2 of the 3 patients did not progress during the remainder of the follow-up period). One of the 16 patients with tumor ≤ 3.5 cm and 6 of the 15 patients with tumor > 3.5 cm experienced tumor recurrence (recurrence rates of 6.3% and 40%, respectively). The group of patients whose tumor size was > 3.5 cm had a higher (p = 0.037) local recurrence rate than the group with tumors ≤ 3.5 cm.
The median follow-up duration post-MWA was 11.1 months (range, 4–32 months). One-year overall survival rate was 44.3%. Median overall survival time was 12 months (95% confidence interval: 8.6–15.4 months) (Fig. 4A). Median local tumor progression-free survival time was 9 months. Local tumor progression-free survival rate was 77.4% (Fig. 4B).
Pain was the most common side effect during the procedures. In these cases, the procedure was completed after the pain was treated. Moderate pain was experienced in 11 sessions and severe pain occurred in 3 sessions (Table 2). The incidence of moderate and severe pain was 38.9% (14/36). When severe pain occurred, the procedure was stopped and patients were treated with morphine injection and midazolam. After MWA, moderate pain was experienced in 4 sessions (11.1%, 4/36). No severe pain occurred after MWA. Eleven patients experienced post-ablation syndrome. Main symptoms were fever (under 38.5℃), fatigue, general malaise, nausea, and vomiting.
During the procedure, patient's BP was increased in 14 sessions (38.9%), including two sessions (5.6%) in which the patient experienced hypertensive crisis. For general increases in BP, nitroglycerin was used to maintain BP at 120–130/80–90 mm Hg. A general increase in BP did not affect the procedure. The procedure was stopped immediately if the patient's systolic pressure was increased beyond 200 mm Hg or diastolic pressure was increased beyond 110 mm Hg with symptoms of hypertensive crisis such as cephalalgia, palpitation, and dyspnea. These patients were treated with phentolamine and nitroglycerin. The procedure was continued when the BP was controlled and maintained at 120–130/80–90 mm Hg. For both patients who experienced hypertensive crisis, the procedure was continued after successfully treating the BP. In one (2.8%) session, local retroperitoneal hematoma was observed. However, CT scanning found that the hematoma was not enlarged at 24 h after the procedure. No other severe complications such as hemorrhoea, intestinal fistula, pancreatic fistula, or adrenal failure occurred in any patient. No patient died during the procedure or within 30 days after MWA.
Image-guided thermal ablation such as radiofrequency ablation (RFA), cryoablation, and MWA is minimally invasive treatment method for local control of tumors. Cryoablation is one of the methods using thermal ablation (19). This method induces cell death by using alternating cycles of freezing and thawing through multiple mechanisms (819). One of the advantages of cryoablation compared to RFA and MWA is that it causes less periprocedural pain. However, cryoablation increases the risk of hemorrhage due to microvascular thrombosis and the inability to coagulate tissue during probe withdrawal (8).
Radiofrequency ablation is one of the most widely applied methods. It represents an effective thermal ablation treatment for adrenal glands (202122). Compared to RFA, MWA has several advantages, including the ability to ablate larger volumes of necrosis with a shorter procedure time, a lessened "heat sink" effect that enables better treatment of the perivascular tissue, the ability to maximize the ablation zone size by positioning multiple MWA antennas into a larger lesion simultaneously, and no requirement for ground pads (23242526). Because of these advantages, more patients with malignancies have received MWA treatment as an alternative option. MWA generally uses either 915 MHz or 2450 MHz. In a microwave electromagnetic field, water molecules, protein molecules, and other polar molecules within the tumor tissue will vibrate at high speeds, resulting in collision and mutual friction between molecules. This can produce temperatures of 60–150℃ in a short time, leading to coagulation necrosis of cells (9101116).
The prognosis of metastatic non-small cell lung cancer (NSCLC) is poor. Standard treatment for this metastatic cancer involves systemic chemotherapy. Platinum-based chemotherapy is recommended as the first-line therapy in most cases, although it improves the median survival time by only a few months (27). One study has found that 7% of patients with oligometastatic cancer may benefit from surgical resection of the primary tumor and its metastasis (28). Several studies (such as Luketich and Burt (29)) have documented the benefit of surgical resection of adrenal metastasis from NSCLC with significantly longer survival time compared to conservative approach. In addition, Moreno et al. (30) have shown that the median overall survival time for the operative treatment of adrenal metastases from NSCLC is 26 months. Porte et al. (31) have also reported that the median survival time is 11 months with 3 of 43 NSCLC patients having survival time of more than 5 years after resection of isolated adrenal metastasis. Compared to surgical resection, conventional external beam radiotherapy has been considered as unreliable for the management of solitary adrenal metastases because treatment responses are typically transient and incomplete (32). Soejima et al. (33) have reported a survival time of 6 months in a study of 14 patients with adrenal metastases receiving radiation.
In our study, according to the size of the 31 lesions in 31 lung cancer patients with solitary adrenal metastases, different numbers of antennas for MWA treatment (a single antenna for lesions ≤ 3.5 cm and two antennas for lesions > 3.5 cm) were chosen. In this study, complete ablation was achieved in 28 (90.3%) of 31 lesions. For tumors ≤ 3.5 cm, the complete ablation rate was 100%, suggesting that MWA is effective for treating solitary adrenal gland metastases from lung cancer. Li et al. (34) and Wang et al. (35) have also reported similar results. The present study included four lesions ≥ 5.0 cm. Complete ablation was not achieved initially in two of these cases. Therefore, a second MWA was performed and complete ablation was ultimately achieved. This suggests that if the lesion is ≥ 5.0 cm, it might be difficult to achieve complete local ablation even with two microwave antennas. Such lesion might require fractional MWA.
Local progression after ablation is one of the most difficult problems in clinical therapy. In this study, progression occurred in 7 (22.6%) of 31 lesions. Of the 7 recurring lesions, only 1 had a maximum diameter of ≤ 3.5 cm. The other 6 recurrent lesions were > 3.5 cm. Thus, the local recurrence rate for lesions with diameter ≤ 3.5 cm was significantly (p = 0.037) lower than that for lesions with diameter > 3.5 cm, suggesting that tumor size is an important factor for local progression. Patients who experienced local progression had the opportunity to achieve ablation with subsequent MWA (for example, Patient No. 7) (Fig. 2).
Based on follow-up results of this study, we found that the median overall survival time was 12 months and the 1-year overall survival rate was 44.3%. The median local tumor progression-free survival time was 9 months and local tumor progression-free survival rate was 77.4%, suggesting that MWA was effective in improving the survival of patients with solitary adrenal gland metastases from lung cancer.
Regarding the safety of MWA, no patient died during the procedure or within 30 days after MWA. The most common side effects in this study were pain and post-ablation syndrome. Minor complications including mild hypertension (38.9% incidence) and local retroperitoneal hematoma (2.8% incidence) were observed. The only major complication observed was hypertensive crisis (5.6% incidence). This might be due to massive release of catecholamines by adrenal medulla in response to high temperature stimulation (36). Hypertensive crisis is a critical complication during MWA of metastatic adrenal neoplasms. It must be treated immediately to prevent cardiocerebral events. When hypertensive crisis occurred, the procedure was stopped immediately and patients were treated with phentolamine and nitroglycerin. After the BP was controlled and maintained at 120–130/80–90 mm Hg, the procedure was continued. No other severe complications such as hemorrhoea, intestinal fistula, pancreatic fistula, or adrenal failure were observed in any patient.
Our study has several limitations. It is retrospective in design with small sample size, short follow-up period, and heterogeneous patient characteristics in terms of cancer stage/histology/adjuvant therapeutic plans. Future studies should employ a larger sample size with these variations controlled. Additional systemic treatment should be included to compare MWA against surgical resection, radiotherapy, or chemotherapy with a prospective, randomized, and controlled study design.
In conclusion, MWA may be fairly safe and effective for treating solitary adrenal gland metastasis from lung cancer. Many issues such as whether MWA can replace surgery and how the efficacy of the treatment could be increased by combining MWA with other treatment methods need to be addressed. Therefore, properly designed, randomized, prospective, and multi-center clinical trials are needed in the future in order to further confirm our results.
Figures and Tables
Fig. 1
Images of 76-year-old woman (patient No. 23) who developed left adrenal metastasis 5 months after lung adenocarcinoma.
A. Contrast-enhanced CT scan showing tumor in left adrenal gland. B. Microwave antenna was placed in tumor. C. One month after ablation, lesion region was larger with lower density without enhancement. D. Twelve months after ablation, lesion region was slightly shrank with lower density without enhancement. On follow-up CT at 3, 6, and 9 months after ablation, lesion region had lower density without enhancement.
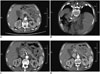
Fig. 2
Images of 61-year-old man (patient No. 10) who developed left adrenal metastasis 3 months after lung adenocarcinoma.
A. CT scan showing tumor (5 cm) in left adrenal gland. B. Microwave antenna was placed in tumor. C. One month after ablation, bottom of lesion region was enhanced. D. For second ablation, microwave antenna was placed in residual tumor. E. One month after second ablation, lesion region had lower density without enhancement. F. At twelve months after ablation, lesion region was slightly shrank with lower density without enhancement.
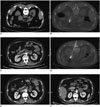
Fig. 3
Images of 54-year-old man (patient No. 11) who developed left adrenal metastasis 12 months after lung adenocarcinoma.
A. Contrast-enhanced CT scan shows tumor in left adrenal gland. B. Microwave antenna was placed in tumor. C. At one month after ablation, lesion region had lower density without enhancement, indicating complete ablation. D. At 30 months after ablation, lesion region was slightly shrank with lower density without enhancement.
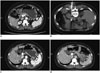
Fig. 4
Overall survival after computed tomography-guided percutaneous microwave ablation of solitary adrenal gland metastasis from lung cancer (A). Local tumor progression-free survival after computed tomography-guided percutaneous microwave ablation of solitary adrenal gland metastasis from lung cancer (B).

Table 1
Patient and Tumor Characteristics and Treatment Summary for Adrenal Metastases in 31 Patients
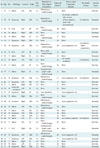
Table 2
Complications of Microwave Ablation (MWA) for Adrenal Metastases from Lung Cancer

Acknowledgments
We express our sincere thanks to Minyong Han, Xiaoying Han, Wenhong Li, Jiao Wang, Xiang Ni, and Yang Ni from the Department of Oncology, Shandong Provincial Hospital Affiliated with Shandong University for his assistance in writing this paper.
References
1. Yamakado K. Image-guided ablation of adrenal lesions. Semin Intervent Radiol. 2014; 31:149–156.
2. Bradley CT, Strong VE. Surgical management of adrenal metastases. J Surg Oncol. 2014; 109:31–35.
3. Vazquez BJ, Richards ML, Lohse CM, Thompson GB, Farley DR, Grant CS, et al. Adrenalectomy improves outcomes of selected patients with metastatic carcinoma. World J Surg. 2012; 36:1400–1405.
4. Yamakado K, Anai H, Takaki H, Sakaguchi H, Tanaka T, Kichikawa K, et al. Adrenal metastasis from hepatocellular carcinoma: radiofrequency ablation combined with adrenal arterial chemoembolization in six patients. AJR Am J Roentgenol. 2009; 192:W300–W305.
5. Mayo-Smith WW, Dupuy DE. Adrenal neoplasms: CT-guided radiofrequency ablation--preliminary results. Radiology. 2004; 231:225–230.
6. Xiao YY, Tian JL, Li JK, Yang L, Zhang JS. CT-guided percutaneous chemical ablation of adrenal neoplasms. AJR Am J Roentgenol. 2008; 190:105–110.
7. Murphy KP, Maher MM, O'Connor OJ. Abdominal ablation techniques. AJR Am J Roentgenol. 2015; 204:W495–W502.
8. Ahmed M, Solbiati L, Brace CL, Breen DJ, Callstrom MR, Charboneau JW, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. J Vasc Interv Radiol. 2014; 25:1691–1705.e4.
9. Ward RC, Healey TT, Dupuy DE. Microwave ablation devices for interventional oncology. Expert Rev Med Devices. 2013; 10:225–238.
10. Abbas G. Microwave ablation. Semin Thorac Cardiovasc Surg. 2011; 23:81–83.
11. Ye X, Fan W, Chen JH, Feng WJ, Gu SZ, Han Y, et al. Chinese expert consensus workshop report: guidelines for thermal ablation of primary and metastatic lung tumors. Thorac Cancer. 2015; 6:112–121.
12. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5:649–655.
13. Ong CK, Lirk P, Seymour RA, Jenkins BJ. The efficacy of preemptive analgesia for acute postoperative pain management: a meta-analysis. Anesth Analg. 2005; 100:757–773. table of contents.
14. Yang X, Ye X, Zheng A, Huang G, Ni X, Wang J, et al. Percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: clinical evaluation of 47 cases. J Surg Oncol. 2014; 110:758–763.
15. Wei Z, Ye X, Yang X, Huang G, Li W, Wang J, et al. Microwave ablation plus chemotherapy improved progression-free survival of advanced non-small cell lung cancer compared to chemotherapy alone. Med Oncol. 2015; 32:464.
16. Lubner MG, Brace CL, Hinshaw JL, Lee FT Jr. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol. 2010; 21:8 Suppl. S192–S203.
17. Crocetti L, Bozzi E, Faviana P, Cioni D, Della Pina C, Sbrana A, et al. Thermal ablation of lung tissue: in vivo experimental comparison of microwave and radiofrequency. Cardiovasc Intervent Radiol. 2010; 33:818–827.
18. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009; 20:7 Suppl. S377–S390.
19. Beland MD, Mayo-Smith WW. Ablation of adrenal neoplasms. Abdom Imaging. 2009; 34:588–592.
20. Ethier MD, Beland MD, Mayo-Smith W. Image-guided ablation of adrenal tumors. Tech Vasc Interv Radiol. 2013; 16:262–268.
21. Pua BB, Solomon SB. Ablative therapies in adrenal tumors: primary and metastatic. J Surg Oncol. 2012; 106:626–631.
22. Uppot RN, Gervais DA. Imaging-guided adrenal tumor ablation. AJR Am J Roentgenol. 2013; 200:1226–1233.
23. Brace CL. Radiofrequency and microwave ablation of the liver, lung, kidney, and bone: what are the differences? Curr Probl Diagn Radiol. 2009; 38:135–143.
24. Carrafiello G, Laganà D, Mangini M, Fontana F, Dionigi G, Boni L, et al. Microwave tumors ablation: principles, clinical applications and review of preliminary experiences. Int J Surg. 2008; 6 Suppl 1. S65–S69.
25. Fan W, Li X, Zhang L, Jiang H, Zhang J. Comparison of microwave ablation and multipolar radiofrequency ablation in vivo using two internally cooled probes. AJR Am J Roentgenol. 2012; 198:W46–W50.
26. Poulou LS, Botsa E, Thanou I, Ziakas PD, Thanos L. Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol. 2015; 7:1054–1063.
27. Spiro SG, Rudd RM, Souhami RL, Brown J, Fairlamb DJ, Gower NH, et al. Chemotherapy versus supportive care in advanced non-small cell lung cancer: improved survival without detriment to quality of life. Thorax. 2004; 59:828–836.
28. Plönes T, Osei-Agyemang T, Krohn A, Passlick B. Surgical treatment of extrapulmonary oligometastatic non-small cell lung cancer. Indian J Surg. 2015; 77:Suppl 2. 216–220.
29. Luketich JD, Burt ME. Does resection of adrenal metastases from non-small cell lung cancer improve survival? Ann Thorac Surg. 1996; 62:1614–1616.
30. Moreno P, de la Quintana Basarrate A, Musholt TJ, Paunovic I, Puccini M, Vidal O, et al. Adrenalectomy for solid tumor metastases: results of a multicenter European study. Surgery. 2013; 154:1215–1222. discussion 1222-1223.
31. Porte H, Siat J, Guibert B, Lepimpec-Barthes F, Jancovici R, Bernard A, et al. Resection of adrenal metastases from non-small cell lung cancer: a multicenter study. Ann Thorac Surg. 2001; 71:981–985.
32. Desai A, Rai H, Haas J, Witten M, Blacksburg S, Schneider JG. A retrospective review of cyberKnife stereotactic body radiotherapy for adrenal tumors (primary and metastatic): Winthrop University Hospital experience. Front Oncol. 2015; 5:185.
33. Soejima T, Hirota S, Hishikawa Y, Hamanaka A, Ozawa Z, Endo M, et al. [Radiation therapy for adrenal metastases]. Nihon Igaku Hoshasen Gakkai Zasshi. 1997; 57:801–804.
34. Li X, Fan W, Zhang L, Zhao M, Huang Z, Li W, et al. CT-guided percutaneous microwave ablation of adrenal malignant carcinoma: preliminary results. Cancer. 2011; 117:5182–5188.
35. Wang Y, Liang P, Yu X, Cheng Z, Yu J, Dong J. Ultrasound-guided percutaneous microwave ablation of adrenal metastasis: preliminary results. Int J Hyperthermia. 2009; 25:455–461.
36. Tsoumakidou G, Buy X, Zickler P, Zupan M, Douchet MP, Gangi A. Life-threatening complication during percutaneous ablation of adrenal gland metastasis: Takotsubo syndrome. Cardiovasc Intervent Radiol. 2010; 33:646–649.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download