Abstract
Objective
Outcomes of stent-assisted coil embolization (SACE) have not been well established in the setting of vertebrobasilar dissecting aneurysms (VBDAs) due to the low percentage of cases that need treatment and the array of available therapeutic options. Herein, we presented clinical and radiographic results of SACE in patients with VBDAs.
Materials and Methods
A total of 47 patients (M:F, 30:17; mean age ± SD, 53.7 ± 12.6 years), with a VBDA who underwent SACE between 2008 and 2014 at two institutions were evaluated retrospectively. Medical records and radiologic data were analyzed to assess the outcome of SACE procedures. Cox proportional hazards regression analysis was conducted to determine the factors that were associated with aneurysmal recanalization after SACE.
Results
Stent-assisted coil embolization technically succeeded in all patients. Three cerebellar infarctions occurred on postembolization day 1, week 2, and month 2, but no other procedure-related complications developed. Immediately following SACE, 25 aneurysms (53.2%) showed no contrast filling into the aneurysmal sac. During a mean follow-up of 20.2 months, 37 lesions (78.7%) appeared completely occluded, whereas 10 lesions showed recanalization, 5 of which required additional embolization. Overall recanalization rate was 12.64% per lesion-year, and mean postoperative time to recanalization was 18 months (range, 3–36 months). In multivariable analysis, major branch involvement (hazard ratio [HR]: 7.28; p = 0.013) and the presence of residual sac filling (HR: 8.49, p = 0.044) were identified as statistically significant independent predictors of recanalization. No bleeding was encountered in follow-up monitoring.
Conclusion
Stent-assisted coil embolization appears feasible and safe for treatment of VBDAs. Long-term results were acceptable in a majority of patients studied, despite a relatively high rate of incomplete occlusion immediately after SACE. Major branch involvement and coiled aneurysms with residual sac filling may predispose to recanalization.
Vertebrobasilar dissection (VBD) is a dynamic process that is variably manifested in angiographic studies as stenosis, occlusion, and aneurysm formation. The reported incidence of vertebrobasilar dissecting aneurysm (VBDA), is as high as 64.9% in patients with unruptured intracranial VBD (1). With the advances in endovascular technologies, reconstruction of VBDAs through stent-assisted coil embolization (SACE) or stent-only deployment is increasing. However, outcomes of SACE in VBDAs have yet to be properly analyzed due to the limited number of patients needing treatment and the array of therapeutic modalities used such as mixed SACE and stent only (23) or mixed reconstructive and deconstructive methods (45). The purpose of this study was to investigate outcomes of SACE in patients with VBDAs and assess risk factors for aneurysmal recanalization after embolization.
A retrospective analysis was conducted, reviewing 66 patients with VBDA treated between January, 2008 and May, 2014 at two affiliated hospitals of Seoul National University (Seoul National University Hospital and Seoul National University Bundang Hospital). This study was approved by the Institutional Review Boards of both facilities. All patients underwent magnetic resonance imaging and catheter angiography before the endovascular procedures, except for ruptured cases that underwent computed tomography angiography (CTA).
Stent-assisted coil embolization was conducted in cases that met the following criteria: 1) focal enlargement of arterial trunk and external vessel diameter with or without the pearl-and-string sign in patients with acute clinical neurologic symptoms that were relevant to VBDA (67); 2) asymptomatic patients with non-saccular aneurysms that showed intramural hematoma and double-lumen sign on CTA or magnetic resonance angiography (MRA) (89). Exclusion criteria were as follows: 1) aneurysm of basilar tip; 2) typical saccular shape; 3) VBDA with concomitant extracranial dissection; 4) concurrent major trauma; and 5) angiitis or vasculopathy confirmed by laboratory diagnostics (1). Trauma in the month prior to dissection was considered major if a medical visit or hospitalization occurred. All other instances were deemed minor trauma (6).
As stipulated by our treatment policy, VBDAs presenting with hemorrhage were primarily treated by deconstructive endovascular means. In any aneurysm arising from a dominant vertebral artery (VA) with poor collaterals or involving arterial branches such as anterior inferior cerebellar artery (AICA) or posterior inferior cerebellar artery (PICA), reconstructive methods were used. Unruptured VBDAs were first treated conservatively. Treatment was indicated for aneurysms that expanded in size during follow-up examinations (n = 5), severe pulsatile posterior headache or neck pain of sudden onset (n = 27), displayed mass effect (n = 1) or recurrent ischemic events (n = 7). Asymptomatic patients who feared rupture and young or middle-aged patients with large-sized VBDAs underwent reconstructive endovascular treatment after appropriate discussion (n = 6). Regarding endovascular treatment, SACE was considered first; however, if technically infeasible, stent-only technique was performed. In our cohort, internal trapping and stentonly were done for 12 and 7 subjects, respectively. As a result, a total of 47 patients with 47 VBDAs, including 30 men and 17 women, were analyzed in this study (Fig. 1). The mean age ± standard deviation (SD) was 53.7 ± 12.6 years. Patient variables extracted from medical records included gender, age, comorbidities (hypertension, diabetes mellitus, hyperlipidemia, and coronary artery disease), smoking history, previous minor trauma, and presenting symptoms. For our purpose, VBDAs were stratified by angiographic features into circumferential and eccentric types (Fig. 2). In circumferential aneurysms, the sac encompasses parent artery by > 180° in three-dimensional images, with or without stenosis and dilatation of parent vessel (10). Eccentric VBDAs were characterized by lateral dilatation. Dissection length was estimated by measuring the distance otherwise normally seen in angiographic views.
In each instance, procedures were performed under general anesthesia, administering antiplatelet medication beforehand. Our institutional protocol calls for dual antiplatelet agent use (clopidogrel and aspirin) (11). In poor responders to clopidogrel, signaled by VerifyNow P2Y12 assay (12), cilostazol was added. A bolus of heparin (3000 IU), given upon placement of the femoral arterial sheath, was thereafter sustained by hourly doses (1000 IU); and activated clotting time was monitored each hour. Continuance of the dual antiplatelet therapy was advised for at least 3 months postoperatively, followed by single-agent maintenance for at least 1 year.
No antiplatelet premedication was given to patients with ruptured aneurysms. Systemic heparinization was initiated after achieving adequate aneurysm protection. Following the procedure, loading dose of clopidogrel and aspirin were administered, and a maintenance regimen (as above) was prescribed.
In our cohort, Enterpriseκ (Codman, Raynham, MA, USA) were utilized exclusively. An Integris V (Philips Medical Systems, Best, the Netherlands) or Innova IGS 630 (GE Healthcare, Milwaukee, WI, USA) scanner was employed in each procedure.
Immediately after embolization, patients were divided into 2 groups, coiled aneurysms with or without initial angiographic residual sac filling, based on the Raymond classification (13). Absence of residual VBDA filling by contrast medium or minimal residual contrast filling at lesion base constituted coiled aneurysms without residual sac filling. Follow-up radiologic tests were done using time-of-flight magnetic resonance angiography (TOF-MRA) or angiography at 6, 12, 24, and 36 months, as dictated by clinical patient status (141516). Plain radiographs were taken at 1 and 3 months after the procedure to view coil shape in VBDAs presenting with hemorrhage (11). Anatomic outcomes in follow-up were interpreted as complete occlusion (no flow or no contrast filling of aneurysms on MRA or angiography) or recanalization. Recanalization was defined as minor recanalization (slight filling at neck of aneurysm) or major recanalization (prominent filling of aneurysmal sac) (17). Repeat embolization was advocated for patients showing major recanalization. Radiologic assessments were conducted by 2 qualified neurointerventionists. Clinical outcomes were scored using the modified Rankin Scale (mRS).
Categorical variables were summarized as counts and percentages and continuous data were expressed as mean ± SD. Cox proportional hazards regression was applied for univariable analysis of factors that were associated with recanalization during the follow-up. Subsequently, multivariable analysis was conducted using variables with p ≤ 0.1 in the univariable analysis in order to identify independent risk factors for recanalization of VBDA after SACE. Statistical significance was set at p < 0.05. All computations relied on standard software (SPSS V. 19; SPSS Inc., Chicago, IL, USA).
A total of 47 patients, each with 1 VBDA, were examined during a mean follow-up period of 20.2 months (range, 3–84 months). The following initial presenting symptoms were observed: subarachnoid hemorrhage (SAH) (7/47, 14.9%), ischemia (6/47, 12.8%), and non-ischemia (34/47, 72.3%). Non-ischemic symptoms included expanding size of aneurysm (n = 5), acute headache (n = 17) or neck pain (n = 5), mass effect (n = 1) or absence of symptoms (n = 6). The mean size and dissection length were 7.8 mm (range, 3.7–18.2 mm) and 6.7 mm (range, 2.5–18.8 mm), respectively. Circumferential (7/47, 14.9%) and eccentric (40/47, 85.1%) subsets were recorded. A total of 16 (34.0%) dissecting aneurysms incorporated major branch of PICA or AICA (Fig. 3). Single stent and double stents were used in 39 (83.0%) and 8 (17.0%) patients, respectively (Table 1).
Stent-assisted coil embolization showed technical success in all patients. For patients with circumferential aneurysms, down-the-barrel view was used during procedure to confirm patency of parent artery (Fig. 4). Immediately following SACE, 25 lesions (53.2%) were successfully occluded without contrast filling into the aneurysmal sac. The other 22 lesions (46.8%) showed contrast filling of the residual sac, with 12 progressing to occlusion (Fig. 5). Overall, 37 lesions (78.7%) were successfully occluded, with major (n = 6) or minor (n = 4) recanalization of 10 lesions in a follow-up period of 79.1 lesion-years. Overall recanalization rate was 12.64% per lesion-year, and mean postoperative time to recanalization was 18 months (range, 3–36 months). Five of the 10 lesions underwent additional procedures (trapping, n = 1; SACE, n = 4). Univariable analysis linked major branch involvement (p = 0.051), circumferential type (p = 0.033), residual sac filling (p = 0.023) with VBDA recanalization after SACE. Maximum size (p = 0.071) and the number of stent (p = 0.093) did not reach statistical significance. In multivariable analysis, major branch involvement (hazard ratio [HR], 7.28; 95% confidence interval [CI] 1.53–34.59; p = 0.013) and residual sac filling (HR, 8.49; 95% CI 1.06–67.70; p = 0.044) were significantly independently associated with recanalization (Table 2).
There were no SACE-related complications; however, 3 cerebral infarctions occurred on day 1, week 2, and month 2 after SACE. One of them presented with SAH and the remaining 2 presented with headache and neck pain without evidence of hemorrhage. Both patients recovered without permanent neurologic sequelae. No intracranial hemorrhage or in-stent re-stenosis was observed during the follow-up period. Most of the patients (n = 46, 97.9%) had excellent clinical outcome (mRS = 0, 44; mRS = 1, 2); however, 1 patient experienced a middle cerebral infarct and post-traumatic hydrocephalus, which were unrelated to the procedure (mRS = 3).
Outcomes of SACE in patients with VBDA remains undetermined as yet, due to the array of treatment modalities and anatomic locations involved (mixed intracranial carotid and vertebrobasilar arterial dissection) (1018). In addition, interpretation of results has been questioned (Supplementary Table 1 in the online-only Data Supplement) (23471819202122). Deconstructive procedures, such as trapping of parent vessel and proximal artery occlusion may be effective in VBDAs that present with hemorrhage. However, ischemic presentations involving these arterial territories or lesions with non-ischemic symptoms are of major concern. In particular, dissecting aneurysms exhibiting saccular incorporation of arterial branches and poor collateral supply are often challenging to treat. Protective SACE at rupture points may be considered in such circumstances. Ro and Kageyama (23) determined a single rupture point in every VA dissection, allowing maximal adventitial extension. Accordingly, protective embolization at rupture points (maximally dilated) offers an alternative to deconstructive treatment in VBDAs presenting with hemorrhage. In our cohort, 7 ruptured VBDAs were treated via SACE. No rebleeding episodes occurred during follow-up, given the protection afforded by SACE, although incomplete occlusion was evident at post-operative angiography.
Utilizations of multiple overlapping stents with coiling have shown good clinical outcomes in patients with dissecting aneurysms. Suh et al. (21) treated 11 cases of ruptured VBDAs using SACE followed by a stent-within-a-stent. In their study, 9 patients showed complete occlusion without in-stent restenosis or perforating arteries occlusion. Zhao et al. (22) also reported that SACE using 2–4 multiple overlapping stents significantly decreased VA dissecting aneurysm recurrence than SACE using single stent (n = 1, 2.1% in multiple stents vs. n = 9, 18.0% in single stent; p = 0.01). In our cohort, SACE was conducted using 1 (n = 39, 83.0%) or 2 stents (n = 8, 17.0%); however, the number of stents (single vs. double) was not associated with recurrence of VBDA in this study.
Preservation of a parent artery can be technically challenging, particularly in VBDAs of circumferential type. In such circumstances, a down-the-barrel view can be useful to confirm patency of a parent vessel (24). If this is not possible, a view delineating most of the aneurysm as it arises from parent vessel may be utilized (24). We also applied conventional coil embolization technique to fusiform dissecting aneurysms under guidance of down-the-barrel views. Compromise of parent artery did not occur during such procedures, despite use of small-sized coil (≤ 2 mm).
Successful occlusion of VBDAs could frequently be difficult if configurations are complex. Previous reports indicate a recurrence rate of 20% in wide-neck intracranial aneurysm (25) and a recanalization rate of 17% in cranial fusiform aneurysms involving anterior and posterior circulations (18). A recanalization rate of 14.3% was noted in one analysis of 7 VBDAs, although 2 patients experienced regrowth and 1 lacked follow-up angiography (18). The presence of major branch involvement of PICA or AICA (p = 0.013) and residual sac filling (p = 0.044) were identified as significant correlates of VBDA recanalization after SACE (Supplementary Fig. 1 in the online-only Data Supplement). Kim et al. (7) reported a 13% recurrence rate after endovascular reconstructive and deconstructive treatments. In particular, PICA origin involvement (p = 0.013) was the only risk factor for future recanalization. They suspected that persistent blood flow to the unprotected dissecting segments due to the preservation of PICA could contribute to the recanalization. For VA dissecting aneurysms, PICA involvement was also associated with recurrence after SACE (22). Mizutani et al. (2627) reported that dissecting aneurysms showed widespread disruption of internal elastic lamina, which showed a greater extent than angiographic findings. Accordingly, deconstructive treatments could be reasonable to prevent future aneurysm recanalization and hemorrhage in VBDAs with major branch involvement, in certain ruptured cases.
The degree of aneurysm occlusion has been associated with recanalization after endovascular treatments (22). Zhao et al. (22) reported that partially occluded aneurysms experienced significantly higher recanalization rate than those with complete or incomplete occlusion (n = 9, 17.6% vs. n = 1, 2.2%) in symptomatic spontaneous VA dissecting aneurysms. In our series, 22 VBDAs showed contrast filling of the residual sac at post-operative angiography, with 12 progressing to occlusion. Among 10 remaining patients with residual sac filling, 9 patients experienced future recanalization (including 6 of major recanalization) except for one sustained incompletely occluded aneurysm on follow-up. Five of the recanalized lesions were additionally treated.
Another critical issue is the time to recanalization of VBDA after SACE. Overall recanalization rates of intracranial aneurysms generally decrease over time following coil embolization. Ng et al. (28) reported a gradual decline in recanalization rates, from 28% in the first year to 14% in the third year postoperatively; however, the aneurysms they studied were largely saccular. Wakhloo et al. (18) discovered that fusiform aneurysms due to dissection or atherosclerotic change had the potential to recanalize up to 3 years after SACE, suggesting perhaps a biologic predisposition to delayed neovascularization. In our cohort, 4 recanalized cases occurred within the first year, 2 within the second year, and 4 within the third year. Hence, long-term angiographic monitoring is essential.
To date, there is no treatment consensus for asymptomatic VBDA owing to an uncertain natural history in a large population; nevertheless, unruptured VBDA has shown benign clinical course in previous studies. Mizutani (29) showed that most unruptured intracranial dissection has low risk of rupture due to healing process. In their study, major morphological change almost occurred within 2 months. For unruptured, non-ischemic dissections concomitant aneurismal dilatation, enlargement or ruptured cases were noted in 4 (16.7%) or 1 (4.2%), respectively. Kim et al. (1) determined a 9.6% rate of spontaneous healing, with restoration of normal arterial caliber in patients with 83 symptomatic VBDAs. In their study, 70 aneurysms remained stable, with only 5 lesions (6.0%) increasing in size. Unfortunately, full details on size morphology of lesions were not provided (1). Kobayashi et al. (9) also showed that 80.5% of unruptured, non-ischemic VBDAs remained morphologically unchanged during a mean follow-up of 2.9 years. They concluded that either maximum aneurysm size or aneurysm ≥ 10 mm were associated with aneurysm enlargement, which may require interventions. In our cohort, 6 asymptomatic patients underwent SACE. Mean values of aneurysm size and patient age were 9.5 mm (range, 7.2–15.9 mm) and 53.3 years (range, 35–64 years), respectively. Five of these lesions were eccentric type and were readily occluded, without complication. The remaining circumferential VBDA required additional SACE due to recanalization. Consequently, asymptomatic VBDAs of eccentric type that are more technically favorable, especially in young or middle-aged patients, may be successfully treated via SACE, although a size threshold must still be established for gauging treatment, growth, and rupture risk.
Our retrospective investigation has several limitations. First, 20 patients (42.6%) were followed only by TOF-MRA (Supplementary Fig. 2 in the online-only Data Supplement). Although some studies (3031) have shown the feasibility of TOF-MRA to evaluate aneurysm occlusion using digital subtraction angiography (DSA) as the reference, the possibility of the stents causing more artifacts could affect the recurrence rate (32). In our cohort, most of the recurrence was diagnosed by DSA (n = 9, 90%). Accordingly, minor recanalization could be obscured in this setting and should be considered to interpret the results. Second, asymptomatic patients with non-saccular aneurysms were defined as VBDA. In clinical circumstances, differentiation of dissection from other underlying vascular abnormality is difficult in incidental non-saccular aneurysms (9). However, we enrolled patients who showed intramural hematoma and double-lumen sign on CTA or MRA. Nevertheless, such lesions could have healing process of chronic dissection, rather than acute dissection (8). Accordingly, our results could not fully reflect the treatment outcomes of symptomatic VBDAs that have acute dissection. Third, findings here are confined to the domain of the Enterprise stent. Open-cell stents were not used.
In conclusion, SACE may be feasible and safe for treating VBDAs. A majority of our patients showed acceptable long-term results, despite a relatively high rate of incomplete occlusion immediately following SACE. Major branch involvement of PICA or AICA and the presence of contrast filling of the residual sac were angiographic features predictive of VBDA recanalization after SACE. Long-term angiographic monitoring is essential due to the potential for delayed recanalization.
Figures and Tables
Fig. 1
Flow diagram of subjects recruited for study.
AICA = anterior inferior cerebellar artery, PICA = posterior inferior cerebellar artery, SACE = stent-assisted coil embolization, VA = vertebral artery

Fig. 2
Angiographic types of vertebrobasilar dissecting aneurysms.
A. Circumferential type: sac encompasses parent artery by > 180° in three-dimensional image. B. Eccentric type: lateral dilatation.
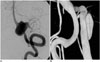
Fig. 3
Illustrative case of ruptured VBDA incorporating major branch of PICA.
A. 76-year-old female presenting with SAH. B. Angiography of right vertebral artery (VA) with proximal sac of fusiform aneurysm incorporating posterior inferior cerebellar artery (PICA) (note hypoplasia of contralateral VA): 6-Fr Envoy guiding catheter placed in distal right VA under general anesthesia. C. Enterprise stent 4.5 × 37 mm deployed across dissecting segment and suspected rupture point (arrows in B and C) successfully protected by coils, without compromise of PICA. SAH = subarachnoid hemorrhage

Fig. 4
Illustrative case of VBDA using down-the-barrel view.
A. 41-year-old female presenting with SAH on CT. B. Angiography of left vertebral artery (VA) showing fusiform aneurysm with medullary branch arising from artery proximal to aneurysm; 6-Fr Envoy guiding catheter placed in distal left VA under general anesthesia and Enterprise stent 4.5 × 28 mm deployed across aneurysm for coil embolization. C. Down-the-barrel view (inner box) used during procedure to confirm patency of parent artery for successful embolization. D, E. Follow-up angiography (postoperative month 12) showing sustained occlusion of aneurysm, without recanalization. SAH = subarachnoid hemorrhage
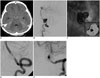
Fig. 5
44-year-old female presenting with severe occipital headache.
A, B. Angiography of right vertebral artery (VA) showing lateral protrusion of aneurysm and mild stenosis proximal to sac; 6-Fr Envoy guiding catheter placed in distal right VA under general anesthesia, two Enterprise stents 4.5 × 22 mm deployed across dissecting segment, and coiling done. C, D. Angiography post-procedure shows residual filling of aneurysm, but follow-up view (postoperative month 12) confirms complete occlusion of sac, without in-stent restenosis.

Table 1
Clinical Characteristics of Subjects with Intracranial Vertebrobasilar Dissecting Aneurysms (n = 47)

Table 2
Risk Factors for Intracranial Vertebrobasilar Dissecting Aneurysm Recanalization after Stent-Assisted Coil Embolization

Acknowledgments
We would like to thank Sung-Eun Kim for her help with the data collection, image preparation and statistical analyses.
References
1. Kim BM, Kim SH, Kim DI, Shin YS, Suh SH, Kim DJ, et al. Outcomes and prognostic factors of intracranial unruptured vertebrobasilar artery dissection. Neurology. 2011; 76:1735–1741.
2. Ahn JY, Han IB, Kim TG, Yoon PH, Lee YJ, Lee BH, et al. Endovascular treatment of intracranial vertebral artery dissections with stent placement or stent-assisted coiling. AJNR Am J Neuroradiol. 2006; 27:1514–1520.
3. Lylyk P, Cohen JE, Ceratto R, Ferrario A, Miranda C. Combined endovascular treatment of dissecting vertebral artery aneurysms by using stents and coils. J Neurosurg. 2001; 94:427–432.
4. Lv X, Jiang C, Li Y, Wu Z. Clinical outcomes of ruptured and unruptured vertebral artery-posterior inferior cerebellar artery complex dissecting aneurysms after endovascular embolization. AJNR Am J Neuroradiol. 2010; 31:1232–1235.
5. Shin GW, Jeong HW. Endovascular treatment of intracranial vertebral artery dissecting aneurysms: follow up angiographic and clinical results of endovascular treatment in serial cases. Neurointervention. 2015; 10:14–21.
6. Debette S, Grond-Ginsbach C, Bodenant M, Kloss M, Engelter S, Metso T, et al. Differential features of carotid and vertebral artery dissections: the CADISP study. Neurology. 2011; 77:1174–1181.
7. Kim BM, Shin YS, Kim SH, Suh SH, Ihn YK, Kim DI, et al. Incidence and risk factors of recurrence after endovascular treatment of intracranial vertebrobasilar dissecting aneurysms. Stroke. 2011; 42:2425–2430.
8. Park SI, Kim BM, Kim DI, Shin YS, Suh SH, Chung EC, et al. Clinical and angiographic follow-up of stent-only therapy for acute intracranial vertebrobasilar dissecting aneurysms. AJNR Am J Neuroradiol. 2009; 30:1351–1356.
9. Kobayashi N, Murayama Y, Yuki I, Ishibashi T, Ebara M, Arakawa H, et al. Natural course of dissecting vertebrobasilar artery aneurysms without stroke. AJNR Am J Neuroradiol. 2014; 35:1371–1375.
10. Jeon P, Kim BM, Kim DI, Park SI, Kim KH, Kim DJ, et al. Reconstructive endovascular treatment of fusiform or ultrawide-neck circumferential aneurysms with multiple overlapping enterprise stents and coiling. AJNR Am J Neuroradiol. 2012; 33:965–971.
11. Cho YD, Lee WJ, Kim KM, Kang HS, Kim JE, Han MH. Stent-assisted coil embolization of posterior communicating artery aneurysms. AJNR Am J Neuroradiol. 2013; 34:2171–2176.
12. Kang HS, Han MH, Kwon BJ, Jung C, Kim JE, Kwon OK, et al. Is clopidogrel premedication useful to reduce thromboembolic events during coil embolization for unruptured intracranial aneurysms? Neurosurgery. 2010; 67:1371–1376. discussion 1376.
13. Raymond J, Guilbert F, Weill A, Georganos SA, Juravsky L, Lambert A, et al. Long-term angiographic recurrences after selective endovascular treatment of aneurysms with detachable coils. Stroke. 2003; 34:1398–1403.
14. Cho YD, Han MH, Ahn JH, Jung SC, Kim CH, Kang HS, et al. Simultaneous endovascular treatment of ruptured cerebral aneurysms and vasospasm. Korean J Radiol. 2015; 16:180–187.
15. Cho YD, Rhim JK, Kang HS, Park JJ, Jeon JP, Kim JE, et al. Use of triple microcatheters for endovascular treatment of wide-necked intracranial aneurysms: a single center experience. Korean J Radiol. 2015; 16:1109–1118.
16. Cho YD, Rhim JK, Park JJ, Jeon JS, Yoo RE, Kang HS, et al. Microcatheter looping to facilitate aneurysm selection in coil embolization of paraclinoid aneurysms. Korean J Radiol. 2015; 16:899–905.
17. Cho YD, Lee JY, Seo JH, Lee SJ, Kang HS, Kim JE, et al. Does stent implantation improve the result of repeat embolization in recanalized aneurysms? Neurosurgery. 2012; 71:2 Suppl Operative. ons253–ons259. discussion ons259.
18. Wakhloo AK, Mandell J, Gounis MJ, Brooks C, Linfante I, Winer J, et al. Stent-assisted reconstructive endovascular repair of cranial fusiform atherosclerotic and dissecting aneurysms: long-term clinical and angiographic follow-up. Stroke. 2008; 39:3288–3296.
19. Suh SH, Kim BM, Chung TS, Kim DI, Kim DJ, Hong CK, et al. Reconstructive endovascular treatment of intracranial fusiform aneurysms: a 1-stage procedure with stent and balloon. AJNR Am J Neuroradiol. 2010; 31:155–160.
20. Dabus G, Lin E, Linfante I. Endovascular treatment of fusiform intracranial vertebral artery aneurysms using reconstructive techniques. J Neurointerv Surg. 2014; 6:589–594.
21. Suh SH, Kim BM, Park SI, Kim DI, Shin YS, Kim EJ, et al. Stent-assisted coil embolization followed by a stent-within-a-stent technique for ruptured dissecting aneurysms of the intracranial vertebrobasilar artery. Clinical article. J Neurosurg. 2009; 111:48–52.
22. Zhao KJ, Zhao R, Huang QH, Xu Y, Hong B, Fang YB, et al. The interaction between stent(s) implantation, PICA involvement, and immediate occlusion degree affect symptomatic intracranial spontaneous vertebral artery dissection aneurysm (sis-VADA) recurrence after reconstructive treatment with stent(s)-assisted coiling. Eur Radiol. 2014; 24:2088–2096.
23. Ro A, Kageyama N. Pathomorphometry of ruptured intracranial vertebral arterial dissection: adventitial rupture, dilated lesion, intimal tear, and medial defect. J Neurosurg. 2013; 119:221–227.
24. Fiorella D, Albuquerque FC, Masaryk TJ, Rasmussen PA, McDougall CG. Balloon-in-stent technique for the constructive endovascular treatment of "ultra-wide necked" circumferential aneurysms. Neurosurgery. 2005; 57:1218–1227. discussion 1218-1227.
25. Murayama Y, Nien YL, Duckwiler G, Gobin YP, Jahan R, Frazee J, et al. Guglielmi detachable coil embolization of cerebral aneurysms: 11 years' experience. J Neurosurg. 2003; 98:959–966.
26. Mizutani T, Miki Y, Kojima H, Suzuki H. Proposed classification of nonatherosclerotic cerebral fusiform and dissecting aneurysms. Neurosurgery. 1999; 45:253–259. discussion 259-260.
27. Mizutani T, Kojima H, Asamoto S, Miki Y. Pathological mechanism and three-dimensional structure of cerebral dissecting aneurysms. J Neurosurg. 2001; 94:712–717.
28. Ng P, Khangure MS, Phatouros CC, Bynevelt M, ApSimon H, McAuliffe W. Endovascular treatment of intracranial aneurysms with Guglielmi detachable coils: analysis of midterm angiographic and clinical outcomes. Stroke. 2002; 33:210–217.
29. Mizutani T. Natural course of intracranial arterial dissections. J Neurosurg. 2011; 114:1037–1044.
30. Nakiri GS, Santos AC, Abud TG, Aragon DC, Colli BO, Abud DG. A comparison between magnetic resonance angiography at 3 Teslas (time-of-flight and contrast-enhanced) and flat-panel digital subtraction angiography in the assessment of embolized brain aneurysms. Clinics (Sao Paulo). 2011; 66:641–648.
31. Ferré JC, Carsin-Nicol B, Morandi X, Carsin M, de Kersaint-Gilly A, Gauvrit JY, et al. Time-of-flight MR angiography at 3T versus digital subtraction angiography in the imaging follow-up of 51 intracranial aneurysms treated with coils. Eur J Radiol. 2009; 72:365–369.
32. Jeon JP, Cho YD, Rhim JK, Park JJ, Cho WS, Kang HS, et al. Effect of stenting on progressive occlusion of small unruptured saccular intracranial aneurysms with residual sac immediately after coil embolization: a propensity score analysis. J Neurointerv Surg. 2015; 10. 27. [Epub]. DOI: 10.1136/neurintsurg-2015-011947.
Supplementary Material
The online-only Data Supplement is available with this article at http://dx.doi.org/10.3348/kjr.2016.17.5.801.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download