Abstract
Stenosis of the pancreatico-enteric anastomosis is one of the major complications of pancreaticoduodenectomy (PD). Endoscopic stent placement, has limited success rate as a nonsurgical treatment due to altered gastrointestinal anatomy. Percutaneous treatment is rarely attempted due to the technical difficulty in accessing the pancreatic duct. We reported a case of pancreaticojejunostomy stenosis after PD, in which a pancreatic stent was successfully placed using a rendezvous technique with a dual percutaneous approach.
Advances in surgical technique has led to decreased surgical mortality rate after pancreaticoduodenectomy (PD), but surgical morbidity has not yet decreased (1). Stenosis of the pancreaticojejunostomy (PJ) is a complication of PD that results in acute recurrent pancreatitis in approximately 5% of such patients (2). In general, re-operation is questionable due to operative risks. Therefore, patients with PJ stenosis are usually treated with endoscopic retrograde cholangiopancreatography-related procedures. However, the success rate of the endoscopic treatment is not very high (3) because the stenotic PJ often cannot be reached by an endoscope due to the alteration of the small bowel anatomy.
The percutaneous treatment of stenotic pancreatic duct was reported in patients with chronic pancreatitis (4), but it has been rarely attempted in patients with acute pancreatitis developed after PD. We reported a case of stenotic PJ after PD, in which a plastic pancreatic stent was successfully placed with a dual (transpancreatic and transhepatic) percutaneous approach.
The Institutional Review Board of our hospital approved the study. The requirement for informed consent was waived. A 17-year-old female had undergone laparoscopic pylorus preserving pancreaticoduodenectomy (PPPD) with Roux-en-Y reconstruction due to a solid pseudopapillary tumor of the pancreas. Two years later, she visited our emergency room with abdominal pain and vomiting. She was diagnosed with acute pancreatitis based on elevated pancreatic enzymes and typical CT findings. Conservative treatment resulted in symptom improvement and the patient was discharged a week later. However, the symptoms of acute pancreatitis recurred a month later. Her serum amylase and lipase levels were 1661 U/L and 16650 U/L, respectively. CT scan revealed a swelling of the remnant pancreas with peripancreatic fluid collections (Fig. 1A) and magnetic resonance cholangiopancreatography showed a stenotic PJ (Fig. 1B), which seemed to be the cause of the recurrent pancreatitis.
We decided to place a pancreatic stent. The endoscopic approach was attempted, but accessing the stenotic PJ failed due to the excessive length of the afferent limb that precluded the advancement of the endoscope to the target site. Instead, we tried the percutaneous approach. The intrahepatic bile duct of segment VI was punctured with a 21 G needle under ultrasonography (US) guidance. After the dilatation of the hepatic parenchymal tract, an 8 Fr vascular sheath was placed through the intra- and extrahepatic bile duct into the jejunum. A 5 Fr angiographic catheter (Torcon NB, Cook, Bloomington, IN, USA) and a 0.035" hydrophilic guidewire (Terumo, Tokyo, Japan) were manipulated to select the PJ anastomosis. The mildly dilated pancreatic duct with focal anastomotic stenosis was opacified by the retrograde filling of small amount of contrast dye injected in the afferent limb; however, the advancement of the guidewire into the pancreatic duct failed due to severe PJ stenosis. Subsequently, we performed percutaneous transgastric puncture of the pancreatic duct with a 21 G needle under US and fluoroscopic guidance (Fig. 2A). A 0.014" hydrophilic guidewire (GT glide; Terumo) was introduced and advanced into the jejunum across the stenotic PJ. The guidewire was captured with a snare catheter introduced via the transhepatic access and pulled out of the patient's body to make a wire loop (Fig. 2B). Pre-stent balloon dilation (4 mm in diameter and 4 cm in length; Savvy, Cordis Inc., Miami, FL, USA) was performed through the transhepatic access to facilitate passage of a plastic stent with non-tapered end (Fig. 2C). Pancreatic stenting beyond the puncture site was impossible due to the pancreatic access at the proximal part of the pancreatic duct. Therefore, a 5 Fr angiographic catheter was introduced via the transhepatic access and advanced across the PJ stenosis, and the 0.014" guidewire was exchanged with a 0.035" stiff guidewire (Terumo) and positioned into the upstream pancreatic duct. A plastic stent (7 Fr in diameter and 5 cm in length; Flexima, Boston Scientific, Natick, MA, USA) was introduced over the guidewire and advanced into position so as to cover the PJ stenosis (Fig. 2D). The transhepatic access was maintained with an 8 Fr drainage catheter (Ultrathane; Cook). The patient's symptoms began to improve from the next day and both the serum amylase and lipase values returned to the normal range in 2 weeks after the stent placement. The patient recovered without complications over the following 3 months. We removed the pancreatic stent using a snare catheter and a biliary drainage tube. At 5 years after the procedure, she was symptom-free with normal blood chemistry test results. Follow-up CT showed complete resolution of the pancreatitis (Fig. 3).
Despite recent advances in surgical technique and perioperative management, Whipple's operation and PPPD are challenging procedures with many complications including delayed gastric emptying, bile leakage, abscess, hemorrhage, acute pancreatitis, and fistula. Among these, acute pancreatitis is one of the most serious complications of PD (1). Although rare, lack of proper treatment may cause the patient to progress to necrotizing pancreatitis with significant mortality (5). For anatomical cause of pancreatitis such as PJ stenosis, conservative therapy usually results in disease recurrence. Therefore, symptomatic pancreatic duct stenosis should be corrected whenever possible (1).
Surgical intervention is a definitive treatment option for PJ stenosis. However, re-operation is generally avoided due to possible postoperative re-stenosis, postoperative adhesions or additional physical burdens on patients. Therefore, endoscopic treatment is the most widely employed nonsurgical treatment to address the PJ stenosis. However, contrary to patients with normal anatomy, the success rate of the endoscopic procedure is not satisfactory because excessive looping of the endoscope and/or the excessive length of the afferent limb precludes the advancement of the endoscope to the PJ anastomosis (3). Furthermore, even if the endoscope reaches the proper site, PJ anastomosis is not always identified and retrograde cannulation of the stenotic anastomosis is more difficult. To overcome these limitations, several endoscopists use endoscopic ultrasonography (EUS)-guided rendezvous technique. This technique involves endoscopic puncture of the pancreatic duct and use of single or double balloon endoscope. However, reported technical success rates are inconsistent (25% to 100%) (678). The largest study with more than 20 cases showed a technical success rate of 48% (7).
Percutaneous puncture of the pancreatic duct was the most technically difficult part of our procedure. Recent advances of US facilitate the identification of minimally dilated pancreatic duct and exact access with a fine needle. If the pancreatic duct is opacified on fluoroscopy like in our case, the puncture can be performed more easily with confidence. However, this is likely to be difficult or even impossible in obese patients and/or in patients with gaseous distension of the stomach. After we achieve a guidewire loop by retrieving the guidewire from the transhepatic access, balloon catheter and stent were introduced retrograde to avoid dilation of the pancreatic access. In our case, the PJ stenosis was benign; hence, we selected a plastic stent due to its retrievability. Since the end of a plastic stent is not tapered, there can be some difficulties in crossing the stenosis. Therefore, pre-stenting balloon dilation seems to be mandatory when there is a tight stenosis. We performed stent placement based on previously reported endoscopic strategies (39). The European society of gastrointestinal endoscopy guidelines recommend treatment of the pancreatic duct stricture by placement of plastic stent, with stent removal or exchange within 1 year even in asymptomatic patients to prevent adverse events related to longstanding stent placement (9). Since the access of the pancreatic duct involved only 21 G needle and 0.014" guidewire, and other devices (balloon catheter and stent) were introduced retrograde through the transhepatic access, the puncture needle could be safely removed without the embolization or any other manipulation.
Our procedure was essentially similar to EUS-guided rendezvous technique (3). However, our case highlighted that the endoscopic procedure can be completely replaced by a dual percutaneous approach involving 1) a percutaneous pancreatic approach instead of EUS-guided transgastric pancreatic puncture, and 2) a transhepatic access to afferent loop instead of endoscopic peroral access. Hence, it can be useful in cases where endoscopic procedures fail or cannot be used as in our case wherein the afferent loop was too long for endoscopic access. Percutaneous antegrade pancreatic stent placement was reported in a few patients with chronic pancreatitis (4). However, this procedure requires dilation of the pancreatic access tract, and has been rarely attempted because of potential serious complications such as pancreatic juice leakage and hemorrhage. On the other hand, retrograde pancreatic stent placement using dual percutaneous approach can be safely performed even in patients with acute pancreatitis.
In summary, treatment with pancreatic stent placement via dual percutaneous approach was successful in a patient with recurrent acute pancreatitis after PD. This case demonstrated that percutaneous pancreatic stenting is safe and technically feasible. It can be useful in cases where endoscopic procedures fail or cannot be used. Further analysis of similar cases is necessary for evidence-based recommendations regarding efficacy and safety of the treatment method.
Figures and Tables
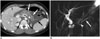 | Fig. 117-year-old patient with recurrent acute pancreatitis after PD.
A. Contrast enhanced CT shows typical features of acute pancreatitis. Note diffuse swelling of remnant pancreas (white arrows) and peripancreatic fluid collection (black arrows). B. MRCP image shows diffuse mild dilatation of pancreatic duct (arrow) and focal stenosis at pancreaticojejunostomy (arrowhead). MRCP = magnetic resonance cholangiopancreatography, PD = pancreaticoduodenectomy
|
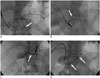 | Fig. 2Radiographic images obtained during pancreatic stent placement.
A. Under fluoroscopy and US guidance, proximal part of pancreatic duct was punctured with 21 G needle (arrow). B. 0.014" guidewire was advanced into jejunum across stenotic PJ (white arrow), which was captured with snare catheter (black arrow). C. Balloon catheter (4 mm in diameter and 4 cm in length, white arrow) was introduced from transhepatic access, and inflated to dilate PJ stenosis. Note focal waist of balloon (black arrow). D. After exchange of guidewire, 7 Fr plastic stent (arrows) was placed to cover the stenosis. PJ = pancreaticojejunostomy, US = ultrasonography
|
References
1. Tani M, Kawai M, Terasawa H, Ueno M, Hama T, Hirono S, et al. Complications with reconstruction procedures in pylorus-preserving pancreaticoduodenectomy. World J Surg. 2005; 29:881–884.
2. Reid-Lombardo KM, Ramos-De la, Thomsen K, Harmsen WS, Farnell MB. Long-term anastomotic complications after pancreaticoduodenectomy for benign diseases. J Gastrointest Surg. 2007; 11:1704–1711.
3. Itoi T, Kasuya K, Sofuni A, Itokawa F, Kurihara T, Yasuda I, et al. Endoscopic ultrasonography-guided pancreatic duct access: techniques and literature review of pancreatography, transmural drainage and rendezvous techniques. Dig Endosc. 2013; 25:241–252.
4. Mathieson JR, Cooperberg PL, Murray DJ, Dashefsky S, Christensen R, Schmidt N. Pancreatic duct obstruction treated with percutaneous antegrade insertion of a metal stent: report of two cases. Radiology. 1992; 185:465–467.
5. Simianu VV, Ramsey MP, Sherman S, Zyromski NJ. Necrotizing pancreatitis caused by pancreatoduodenectomy. Pancreas. 2010; 39:942–943.
6. Mallery S, Matlock J, Freeman ML. EUS-guided rendezvous drainage of obstructed biliary and pancreatic ducts: Report of 6 cases. Gastrointest Endosc. 2004; 59:100–107.
7. Barkay O, Sherman S, McHenry L, Yoo BM, Fogel EL, Watkins JL, et al. Therapeutic EUS-assisted endoscopic retrograde pancreatography after failed pancreatic duct cannulation at ERCP. Gastrointest Endosc. 2010; 71:1166–1173.
8. Will U, Fueldner F, Thieme AK, Goldmann B, Gerlach R, Wanzar I, et al. Transgastric pancreatography and EUS-guided drainage of the pancreatic duct. J Hepatobiliary Pancreat Surg. 2007; 14:377–382.
9. Dumonceau JM, Delhaye M, Tringali A, Dominguez-Munoz JE, Poley JW, Arvanitaki M, et al. Endoscopic treatment of chronic pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2012; 44:784–800.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download