Abstract
We report multidetector computed tomography (MDCT) and cardiac magnetic resonance (CMR) findings of a 34-year-old female with isolated left ventricular apical hypoplasia. The MDCT and CMR scans displayed a spherical left ventricle (LV) with extensive fatty infiltration within the myocardium at the apex, interventricular septum and inferior wall, anteroapical origin of the papillary muscle, right ventricle wrapping around the deficient LV apex, and impaired systolic function. MDCT visualized morphologic and also functional findings of this unique cardiomyopathy.
Isolated left ventricular apical hypoplasia (LVAH) is a unique congenital cardiomyopathy. To our knowledge, there are only a few case reports on the multidetector computed tomography (MDCT) and cardiac magnetic resonance (CMR) findings of LVAH with fatty infiltration usually confined within the left ventricular apical myocardium. Here we present the morphologic and functional findings of LVAH in a female patient with extensive fat infiltration along the interventricular septum and inferior myocardium. This study was approved by the Institutional Review Board of our hospital.
A 34-year-old woman, with no past medical history of cardiac diseases, was referred to our hospital for an evaluation of an atypical chest discomfort. She was physically active with good exercise tolerance (New York Heart Association class I). Her personal and familial medical histories were noncontributory, and a physical examination found no detectable heart murmur. Electrocardiography (ECG) showed Q wave in leads V1-4, and chest radiography revealed cardiomegaly.
A transthoracic echocardiogram subsequently revealed a globular, thin-walled, and severely dilated left ventricle (LV) with a brightly echogenic apex, bulging of the interventricular septum towards the right ventricle (RV), and a mildly reduced systolic function (ejection fraction [EF], 44%). There was a grade II diastolic dysfunction and elevated left ventricular end-diastolic pressure (Fig. 1A). The presumptive diagnosis was dilated cardiomyopathy with an LV mass at the apex.
The patient underwent a CMR scan and cardiac catheterization for evaluation of ischemic or nonischemic cardiomyopathy with an LV mass. The left heart catheterization revealed a normal coronary artery. CMR imaging was performed using a 1.5T scanner (Intera CV, Philips Medical Systems, Best, the Netherlands) and a dedicated 5-channel phased-array surface cardiac coil. For anatomical evaluation, an axial T2-weighted black-blood sequence (repetition time [TR]/echo time [TE], 1500/100 msec; matrix size, 248 × 210; slice thickness, 6 mm) was acquired. For volumetric and functional imaging, breath-hold standard cine steady-state free-precession sequences in short-axis, 4-chamber view and RV vertical long-axis orientation were acquired (TR/TE, 500/50 msec; matrix size, 176 × 217; slice thickness, 8 mm). Short-axis images covered the whole heart gapless from the apex to the base (20 phases per heart cycle). Phase-sensitive inversion recovery late gadolinium enhancement was acquired in the diastole period, 15 minutes after intravenous injection of gadobenate dimeglumine at 0.1 mmol/kg of body weight (MultiHance, Altana Pharma, Konstanz, Germany). All sequences were executed with ECG-gating and breath-hold technique.
Cardiac magnetic resonance revealed a spherical and truncated LV with rightward bulging of the interventricular septum, as well as prominent fat presence at the apex (Fig. 1B, arrows). The CMR also showed an elongated RV, wrapping around the deficient LV apical area to form the true apex. The papillary muscle originated from the flattened anterior apex. The left-ventricular indexed systolic function and left-ventricular indexed mass were decreased (LVEF, 44%; LV mass index, 35 g/m2). The left ventricular end diastolic volume index (LVEDVI) was increased (LVEDVI, 119 mL/m2). The right-ventricular systolic function was low normal (RVEF, 56%). There was no delayed myocardial enhancement to indicate myocardial fibrosis or mass (Fig. 1B).
A prospectively triggered CT scan was performed, using a 128-slice CT scanner (SOMATOM Definition Flash, Siemens, Erlangen, Germany). Scan parameters were as follows: 2 × 128 × 0.6 sectional collimation; 280 msec gantry rotation time; 100 kVp tube voltage; and 320 mA with the automatic tube current modulation technique. Iodinated contrast medium (Ultravist [iopromide], 300 mg I/mL, Bayer Healthcare, Berlin, Germany) was injected via peripheral veins at a volume of 1.5 mL/kg body weight. Automatic triggering was used. A saline chaser of 1.5 mL/kg body weight was injected at the same rate of contrast medium to reduce artifact which may be caused by contrast medium in the superior vena cava. Images were reconstructed to 0.75 mm in thickness and interval reconstruction with an I30f kernel filter and were transferred to a workstation (RAPIDIA 3D; Infinitt, Seoul, Korea) for post-processing. Images were analyzed in a workstation using multiplanar reformation and endo-cardiovascular volume-rendering (VR) technique. Unlike traditional endo-cast VR of enhanced lumen, the endo-cardiovascular VR was performed by applying trapezoids of transfer function to null-out the enhanced lumen and depict the cardiac wall (Supplementary Video in the online-only Data Supplement). We encoded different colors for fat and cardiac muscle and dissected out the obscuring wall to disclose the internal anatomy of interest.
The extent to which the fatty material replaced the deficient apical myocardium, interventricular septum and LV inferior wall was better seen on an MDCT (Fig. 1C). Almost all the fatty material was confined within the myocardium; only a small portion of the apical fat was contiguous with epicardial fat (Fig. 1C, arrow).
The systolic function of LV was decreased, whereas the right-ventricular systolic function was on the lower end of normal (LVEF, 41%; RVEF, 50%). Impaired systolic function was also demonstrated on 3-dimensional (3D) endo cardiovascular VR cine imaging (Supplementary Video in the online-only Data Supplement).
Our findings from the imaging were consistent with an isolated LV apical hypoplasia. Therefore, the patient was treated with standard therapy for a dilated cardiomyopathy, with an angiotensin converting enzyme inhibitor, and was recommended a close follow-up.
Isolated LVAH is a newly recognized, unusual cardiomyopathy and its clinical course is uncertain. Isolated LVAH was first described in 2004 in a case report by Fernandez-Valls et al. (1). This anomaly may occur as an isolated congenital anomaly or in conjunction with other congenital cardiac abnormalities (12345678). The clinical presentation varies from asymptomatic to symptoms ranging from a minor degree of fatigue, to chest pain, exertional dyspnea, ventricular tachyarrhythmias, biventricular cardiac failure and pulmonary hypertension (49). Therefore, a close follow-up is needed even in asymptomatic patients. The hemodynamic is similar to restrictive LV cardiomyopathy and almost all patients responded well to the standard heart failure treatment (4).
Our diagnosis was made using a transthoracic echocardiogram, CMR and MDCT. The typical four imaging characteristics were: 1) a truncated, spherical LV with bulging of the interventricular septum to RV as there was impaired LV function in the form of impaired late diastolic filling and systolic function; 2) fatty infiltration of the left ventricular apical myocardium; 3) an elongated, normally functioning RV that wraps around the deficient LV apex; and 4) an anteroapical origin of papillary muscle (1).
Our patient presented with atypical chest discomfort during a routine medical examination. MDCT demonstrated a decreased systolic function on the 3D endo-cardiovascular VR dynamic image and CT volumetry. There were prominent adipose tissue infiltrates of the apex, interventricular septum and the inferior wall. This CT finding was unique and, to our knowledge, there have been no case reports demonstrating extensive fatty infiltration along the interventricular septum and inferior myocardium. Besides the rarity of this finding, this reporting may also had benefited from a higher spatial resolution of the MDCT scan.
Fernandez-Valls et al. (1) reported that invagination of fatty material in the defective LV apex from the epicardial fat was seen in all patients. However in our patient, MDCT demonstrated no invagination but infiltration of fatty material within the myocardium, which was contiguous with epicardial fat in some portions.
Although the pathogenesis of this cardiomyopathy is unknown, it is postulated to be a congenital anomaly, due to the abnormal ventricular septation during partitioning. A defective LV apical development might be attributable to a relatively inadequate LV to RV dilatation in the fifth week of embryonic development, leading to a spherical LV with RV elongation wrapping around the LV apex while the interventricular septum continued to develop (1).
Isolated LV apical hypoplasia should be differentiated from idiopathic dilated cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy with left ventricular preponderance, ischemic heart disease, hypoplastic left heart syndrome and non-compaction of the LV. Idiopathic dilated cardiomyopathy is diagnosed by a left or biventricular dilation, with severely impaired systolic function, in the absence of abnormal loading conditions (10). Arrhythmogenic left ventricular cardiomyopathy is characterized by dilatation or the formation of an aneurysm with an associated paradoxical motion, diffuse fatty infiltration and subendocardial enhancement (1112). Hypoplastic left heart syndrome is accompanied by aortic or mitral atresia. Non-compaction of the LV is characterized by a diffusely dilated LV with a heavily trabeculated endocardium. Our patient had mature mitral and aortic valves, normal coronary arteries and no heavily trabeculated endocardium.
To our knowledge, this is the first case of LV apical hypoplasia to demonstrate the diagnostic ability of the ECG-gated MDCT, showing morphologic and also functional characteristics. This allowed demonstrations of an extensive LV apex, interventricular septal and inferior wall fatty replacement, and decreased systolic function. Familiarity with the characteristic imaging findings of this unusual cardiomyopathy is essential for a more prompt diagnosis and accurate treatment.
Figures and Tables
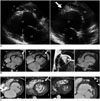 | Fig. 1Isolated left ventricular apical hypoplasia in 34-year-old woman.
A. Echocardiogram demonstrates spherical and truncated LV with echo-bright apex (arrow), bulging of interventricular septum towards right ventricle, and elongated RV wrapping around deficient LV apex in parasternal long-axis view. B. Four-chamber view from CMR during diastole and systole shows spherical, truncated LV at apical level with prominent fat (arrows). Papillary muscles originate from flattened anterior apex. RV is elongated and wraps around deficient LV apex. Late gadolinium enhancement revealed no enhancement in apex and ventricular septum of LV. C. Four-chamber view from cardiac MDCT more clearly visualizes extent of fatty material within apical myocardium, interventricular septum and inferior LV wall. Almost all fatty material was confined within myocardium, although small portion of apical fat was contiguous with epicardial fat (arrow). MDCT clearly shows cardiac layers and fat distribution. Fatty tissue is confined within thin myocardial layers of apex and interventricular septum. Note pericardium (arrowheads) and epicardial fat. CMR = cardiac magnetic resonance, LA = left atrium, LV = left ventricle, MDCT = multidetector computed tomography, RV = right ventricle, VR = volume-rendering
|
References
1. Fernandez-Valls M, Srichai MB, Stillman AE, White RD. Isolated left ventricular apical hypoplasia: a new congenital anomaly described with cardiac tomography. Heart. 2004; 90:552–555.
2. Marin C, Sanchez ML, Maroto E, Ossaba S, Ruiz Y, Zabala JI. MR imaging of isolated left ventricular apical hypoplasia. Pediatr Radiol. 2007; 37:703–705.
3. Flett AS, Elliott PM, Moon JC. Images in cardiovascular medicine. Cardiovascular magnetic resonance of isolated left ventricular apical hypoplasia. Circulation. 2008; 117:e504–e505.
4. Irving CA, Chaudhari MP. Fatal presentation of congenital isolated left ventricular apical hypoplasia. Eur J Cardiothorac Surg. 2009; 35:368–369.
5. Patrianakos AP, Protonotarios N, Zacharaki A, Tsatsopoulou A, Parthenakis FI, Vardas PE. Isolated left ventricular apical hypoplasia: a newly recognized unclassified cardiomyopathy. J Am Soc Echocardiogr. 2010; 23:1336.e1–1336.e4.
6. Haffajee JA, Finley JJ, Brooks EL, Kuvin JT, Patel AR. Echocardiographic characterization of left ventricular apical hypoplasia accompanied by a patent ductus arteriosus. Eur J Echocardiogr. 2011; 12:E17.
7. Moon JI, Jeong YJ, Lee G, Choi JH, Lee JW. Isolated left ventricular apical hypoplasia with infundibular pulmonary and aortic stenosis: a rare combination. Korean J Radiol. 2013; 14:874–877.
8. Chaowu Y, Xin S, Shihua Z, Jianrong L, Hao W. Complete transposition of the atrioventricular valves associated with left ventricular apical hypoplasia. Circulation. 2011; 124:e538–e539.
9. Motwani M, Witte KK, Plein S, Greenwood JP. Isolated left ventricular apical hypoplasia evaluated by cardiovascular magnetic resonance and gadolinium enhancement techniques. J Am Coll Cardiol. 2011; 58:2355.
10. Francone M. Role of cardiac magnetic resonance in the evaluation of dilated cardiomyopathy: diagnostic contribution and prognostic significance. ISRN Radiol. 2014; 2014:365404.
11. Coats CJ, Quarta G, Flett AS, Pantazis AA, McKenna WJ, Moon JC. Arrhythmogenic left ventricular cardiomyopathy. Circulation. 2009; 120:2613–2614.
12. Paetsch I, Reith S, Gassler N, Jahnke C. Isolated arrhythmogenic left ventricular cardiomyopathy identified by cardiac magnetic resonance imaging. Eur Heart J. 2011; 32:2840.
Supplementary Materials
The online-only Data Supplement is available with this article at http://dx.doi.org/10.3348/kjr.2016.17.1.79.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download