This article has been corrected. See "Erratum: Endovascular Repair of Blunt Popliteal Arterial Injuries" in Volume 17 on page 967.
Abstract
Objective
To evaluate the feasibility and effectiveness of endovascular repair for blunt popliteal arterial injuries.
Materials and Methods
A retrospective analysis of seven patients with clinical suspicion of popliteal arterial injuries that were confirmed by arteriography was performed from September 2009 to July 2014. Clinical data included demographics, mechanism of injury, type of injury, location of injury, concomitant injuries, time of endovascular procedures, time interval from trauma to blood flow restoration, instrument utilized, and follow-up. All patients were male (mean age of 35.9 ± 10.3 years). The type of lesion involved intimal injury (n = 1), partial transection (n = 2), complete transection (n = 2), arteriovenous fistula (n = 1), and pseudoaneurysm (n = 1). All patients underwent endovascular repair of blunt popliteal arterial injuries.
Results
Technical success rate was 100%. Intimal injury was treated with a bare-metal stent. Pseudoaneurysm and popliteal artery transections were treated with bare-metal stents. Arteriovenous fistula was treated with bare-metal stent and coils. No perioperative death and procedure-related complication occurred. The average follow-up was 20.9 ± 2.3 months (range 18–24 months). One patient underwent intra-arterial thrombolysis due to stent thrombosis at 18 months after the procedure. All limbs were salvaged. Stent migration, deformation, or fracture was not found during the follow-up.
Lower extremity arterial injuries, especially of the popliteal artery accounting for < 0.2% of all trauma cases, are uncommon but have potentially devastating consequence that cause significant morbidity and mortality (1). A recent study demonstrated that limb salvage of patients with popliteal arterial injuries is influenced by prolonged ischemia time (2). The primary principle in treating acute traumatic popliteal arterial injuries is to avoid prolonged ischemia. This reduces the risk of irreversible ischemia and morbidity associated with ischemia reperfusion (3). Traditional surgical management, as the gold standard for treatment of popliteal arterial injuries (45), remains a challenge due to complex anatomic locations (6). Disadvantages of surgical exploration include the need for anesthesia and risk associated with injured site infection (7). Endovascular repair has been widely employed in the treatment of arterial injuries with the advances in orgtechnology of stent and stent-graft (89101112). Endovascular repair of arterial injuries has theoretical advantages over the surgical approach, including a minimally invasive, rapid procedure, a more rapid recovery time, lower in-hospital mortality, and decreasing risk of injuring important adjacent structures (12131415). Patients with severe concomitant injuries, especially hemorrhagic shock patients, may benefit from endovascular management (16). However, there is little experience with endovascular repair in the management of blunt popliteal artery injuries (17).The purpose of this retrospective study was to describe our experience of endovascular repair of blunt popliteal arterial injuries.
Ethical approval for the study was obtained from the scientific and research ethics board of the hospital.
Clinical data of all patients were retrospectively analyzed from September 2009 to July 2014. Patient selection criteria for endovascular repair included patients who had a clinical suspicion of popliteal arterial injuries and no open injuries of the knee joint, and appropriate access sites were required. Exclusion criteria included pregnant or lactating females and females contemplating pregnancy; patients who had open injuries of the knee joint; children; and contraindication to antiplatelet, anticoagulant, or thrombolysis. Demographics, mechanism of injury, type of injury, location of injury, concomitant injuries, time of endovascular procedures, time interval from trauma to blood flow restoration, instrument utilized, and follow-up were recorded.
All endovascular procedures were performed in an angiography suite using digital subtraction angiography (Angiostar, Siemens, Munich, Germany). All patients received an initial intravenous bolus of (3000–5000) U unfractionated heparin depending on the patients' weight, which was continuously infused at a rate of 1000 U/h. Patients were positioned supine under local anesthesia with 2% lignocaine (10 mL) at vascular access site. Ipsilateral femoral artery was punctured using the Seldinger technique under ultrasound guidance. A 6-Fr sheath (Terumo, Tokyo, Japan) was placed with the assistance of a 0.035-inch hydrophilic guidewire (Radifocus, Terumo, Tokyo, Japan). The severity, location and type of arterial injury were confirmed by arteriography with a 4-Fr vertebral catheter (Cordis, Miami Lakes, FL, USA) from 6-Fr sheath placed in the ipsilateral femoral artery (Figs. 1A, 2A). Injured arteries were crossed via guidewire and vertebral catheter. When partial or complete popliteal artery transection was confirmed by angiography, dual access (femoral and posterior tibial) could be adopted to solve the difficulty of passing through the injured site with guidewire. A goose neck snare (Shanghai Shape Memory Alloy, Shanghai, China) was introduced from the posterior tibial access to snare transfemoral 0.035-inch hydrophilic guidewire and established working wire pathway. Appropriate sized bare-metal stent (LifeStent, Bard Peripheral Vascular, Tempe, AZ, USA) from ipsilateral femoral access over a 0.035-inch super stiff wire with the length of 260 mm (Terumo, Tokyo, Japan) was implanted to manage injured popliteal arteries. The length of the stent was required to span the lesion; the diameter of the stent was measured by intraoperative arteriography with approximately 10% oversizing. The result of arteriography determined further stent deployment after first stent. Stenosed segments of the stent were traversed with a 0.035-inch super stiff wire of 260 mm length from ipsilateral femoral access and dilated using a 5 × 10 mm angioplasty dilatation catheter (Bard Peripheral Vascular, Tempe, AZ, USA) to achieve satisfactory blood flow. The balloon of angioplasty dilatation catheter was inflated to between 4 and 6 atmospheres. A guiding catheter (Cordis, Miami Lakes, FL, USA) through 6-Fr sheath from ipsilateral femoral access was used to aspirate thrombus of distal artery. If residual thrombus was confirmed by angiography, a 4-Fr infusion catheter (AngioDynamics, Queensbury, NY, USA) from ipsilateral femoral access was inserted into the distal artery. A small dose of urokinase ([20−30] × 104 U) was pumped via the infusion catheter into the distal artery for thrombolysis during the procedure. Successful endovascular repair was defined as the restoration of blood flow on arteriography at the end of the procedure. Arteriography confirmed the patency of stents and the adequate distal runoff to the leg (Figs. 1C, D, 2B). Access sites were subsequently wrapped and compressed for 6 hours to achieve hemostasis after removal of the 6-Fr sheath.
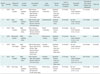
From September 2009 to July 2014, seven male patients who matched the selective criteria received endovascular repair for popliteal arterial injuries. The mean age was 35.9 ± 10.3 years (range 23–53 years). Two patients had left popliteal arterial injuries and five patients had right. Three patients presented hemorrhagic shock on arrival to our hospital. Extremity fracture was present in six patients. The demographic and clinical data for seven patients were presented in Table 1.
All patients with clinical suspicion of popliteal arterial injuries underwent arteriography to confirm the diagnosis. Technical success was observed in all patients with a mean procedural time of 30 minutes (range 20–40 minutes). At the end of the endovascular procedure, angiography presented completely restored blood flow within injured popliteal arteries without contrast media extravasation. In total, eleven stents were deployed in seven patients. Two-layered bare-metal stents were implanted in four patients. All stents were LifeStent (Bard Peripheral Vascular, Tempe, AZ, USA). The length of the stent ranged between 4 and 15 cm; the diameter of the stents was 6 mm. In addition, coils (Cook, Bloomington, IN, USA) were deployed into fistula through the mesh of the stent to close fistula for patient 4 (Fig. 1B). Details of endovascular procedures, mechanism and type of injury to popliteal artery were shown in Table 1. After endovascular procedures, bedside ultrasonography showed patency of the popliteal artery and no access site-related complications. Dorsalis pedis arterial pulses were palpable. One patient required fasciotomy for compartment syndrome after 1 day of endovascular procedure. No perioperative death and procedure-related complication occurred.
The average follow-up was 20.9 ± 2.3 months (range 18–24 months). One patient with absence of dorsalis pedis arterial pulses was referred for intra-arterial thrombolysis due to stent thrombosis confirmed by color Doppler ultrasonography at 18 months after the procedure. The remainder were asymptomatic and had patent stents confirmed by color Doppler ultrasonography or computed tomographic angiography during the follow-up period. All limbs were salvaged. Stent migration, deformation, or fracture was not found during the follow-up.
The popliteal artery is the second most common lower extremity arterial injuries, accounting for 30.7% (18). In World War II, popliteal arterial injuries resulted in 72.5% amputation rate with simple ligation (19). Advanced surgical technique reduces amputation rate to 14.5–21% in civilian vascular trauma (120). The amputation rate is 50% for patients with lower extremity arterial injuries when delayed by > 6 hours in management, as compared with < 10% amputation rate for delays of < 6 hours (21).
The gold standard of treatment is immediate surgical repair when popliteal arterial injuries are suspected (45). Surgical repair is technically challenging, as it is affected by the degree of injury, anatomic distortion around the lesion, excessive bleeding, and has an increased risk of iatrogenic nerve injury (2223). Anatomic variations of the popliteal artery often lead to more difficult surgical reconstruction procedures with increased risk of iatrogenic vascular injury (2425).
Since the first reported case of stent-graft treatment for arterial injuries in 1991 (26), the use of endovascular therapy in vascular injuries has increased dramatically (89101112). Endovascular treatment has lower amputation rates and complications than surgical repair (12). Avoiding operating on injured soft tissue is another potential advantage of endovascular maneuvers (27).
Treatment of transected popliteal artery with endovascular repair remains challenging in emergency situations (28). We preferred dual access (femoral and posterior tibial) to revascularize partial or complete transection of the popliteal artery. The guidewire was introduced through the ipsilateral femoral access and was snared from posterior tibial access with a goose neck snare. A guidewire passed through the transected area by "through-and-through" wire technique and stents were implanted to repair injured arteries. In our series, the injured popliteal artery was successfully repaired by this technique in two patients with complete popliteal artery transaction and one patient with partial transaction. If necessary, thrombus aspiration or thrombolysis may be considered as therapy for distal embolization.
The treatment of popliteal artery lesions with endovascular approach remains controversial (293031). The stent in the popliteal arterial is more vulnerable to mechanical forces, resulting in stent fracture (32). However, crossing the knee joint with a device is not a contraindication (33). Piffaretti et al. (34) described ten patients with peripheral arterial injuries following blunt trauma treated with stent or stent-graft. Although five of ten lesions involved an artery across a joint or arterial regions subjected to strong mechanical forces, no deformity, breakage or migration was noted during the follow-up period. The LifeStent, a nitinol self-expanding stent, could be considered due to this kind of bare-metal stent with relatively good flexibility and lower risk of fracture (35). The expandable type of LifeStent is self-expanding. The chief advantage of self-expanding stents is flexibility, which allows quick deployment in tortuous vessels (36). In addition, bare stent could avoid acute ischemia of side branches of the popliteal artery, as compared to stent-graft. Ruffino and Rabbia (37) reported that the cumulative patency of side branch covered by bare-metal stent was 96.1% in endovascular repair of peripheral and visceral aneurysms. In our study, no popliteal side branch occlusion was noted during the follow-up period. Pieper et al. (38) found a marked reduction of flow outside the normal vessel wall after multilayer stent placement. In our four patients, the bleeding was controlled after two-layered bare-metal stents placement. The membrane material of stent-graft can delay endothelialization of stent-graft. The presence of metal stent may be beneficial to the endothelialization (3940). Additionally, Henry et al. (41) reported the limitations of stent-graft placement in small-diameter arteries. The conclusion obtained from elderly patients with atherosclerosis may be not applicable to traumatic popliteal arterial injury patients. Atherosclerosis is rare in most patients who are young or middle-aged. Bare-metal stents may be preferable to stent-graft for blunt popliteal arterial injuries.
Arteriovenous fistulas can be managed with coil embolization with or without stent (4243). Arteriovenous fistula was diagnosed by angiography in patient 4 who had a history of gunshot wounds for two years and presented lower extremity swelling and varicose veins. A stent was placed into the right popliteal artery to slow the venous flow, which prevented migration of coils. Multiple coils were subsequently placed from the stent mesh into fistula to exclude arteriovenous fistula.
Conventional surgical repair can be performed in the field of injured arteries in cases of stent-related complications. Contraindications for endovascular treatment of blunt popliteal arterial injuries are preoperative compartment syndrome, contrast medium allergy, and access site infection.
However, our study has several limitations. Firstly, the follow-up period is relatively short. Secondly, surgical control patients are absent in this retrospective study. Finally, in order to shorten the time of limb ischemia and increase the chance of limb salvage, patients were diagnosed by angiography that has the function of both diagnosis and treatment, instead of color Doppler ultrasonography or computed tomographic angiography.
In conclusion, endovascular repair seems to be a viable approach for patients with blunt popliteal arterial injuries, especially on an emergency basis. Endovascular repair may be effective in the short-term. Further studies are warranted to evaluate the long-term efficacy of endovascular repair.
Figures and Tables
Fig. 1
Old arteriovenous fistula of right popliteal artery.
A. Selective angiography of right popliteal artery shows arteriovenous fistula. B. Angiography was performed to assess fistula after bare stent (6 mm × 4 cm) implantation. Coils were implanted to fistula through mesh of stent. C, D. Disappearance of arteriovenous fistula was confirmed by angiography.
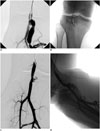
Fig. 2
Transection of right popliteal artery.
A. Selective angiography shows transection of right popliteal artery and proximal end of right popliteal artery was blocked by hematomas. B. Angiography was performed after deployment of two stents (6 mm × 15 cm) with through-and-through wire access.
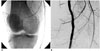
Table 1
Summary of Demographic and Clinical Data of All Patients
References
1. Mullenix PS, Steele SR, Andersen CA, Starnes BW, Salim A, Martin MJ. Limb salvage and outcomes among patients with traumatic popliteal vascular injury: an analysis of the National Trauma Data Bank. J Vasc Surg. 2006; 44:94–100.
2. Lang NW, Joestl JB, Platzer P. Characteristics and clinical outcome in patients after popliteal artery injury. J Vasc Surg. 2015; 61:1495–1500.
3. Huynh TT, Pham M, Griffin LW, Villa MA, Przybyla JA, Torres RH, et al. Management of distal femoral and popliteal arterial injuries: an update. Am J Surg. 2006; 192:773–778.
4. Frykberg ER. Popliteal vascular injuries. Surg Clin North Am. 2002; 82:67–89.
5. Halvorson JJ, Anz A, Langfitt M, Deonanan JK, Scott A, Teasdall RD, et al. Vascular injury associated with extremity trauma: initial diagnosis and management. J Am Acad Orthop Surg. 2011; 19:495–504.
6. Sciarretta JD, Perez-Alonso AJ, Ebler DJ, Mazzini FN, Petrone P, Asensio-Gonzalez JA. Popliteal vessel injuries: complex anatomy, difficult problems and surgical challenges. Eur J Trauma Emerg Surg. 2012; 38:373–391.
7. Scalea TM, Sclafani S. Interventional techniques in vascular trauma. Surg Clin North Am. 2001; 81:1281–1297. xii
8. Carrafiello G, Laganà D, Mangini M, Fontana F, Chiara R, Filippo P, et al. Percutaneous treatment of traumatic upper-extremity arterial injuries: a single-center experience. J Vasc Interv Radiol. 2011; 22:34–39.
9. Onal B, Ilgit ET, Koşar S, Akkan K, Gümüş T, Akpek S. Endovascular treatment of peripheral vascular lesions with stent-grafts. Diagn Interv Radiol. 2005; 11:170–174.
10. Canaud L, Hireche K, Joyeux F, D'Annoville T, Berthet JP, Marty-Ané C, et al. Endovascular repair of aorto-iliac artery injuries after lumbar-spine surgery. Eur J Vasc Endovasc Surg. 2011; 42:167–171.
11. Canaud L, Marty-Ané C, Ziza V, Branchereau P, Alric P. Minimum 10-year follow-up of endovascular repair for acute traumatic transection of the thoracic aorta. J Thorac Cardiovasc Surg. 2015; 149:825–829.
12. Branco BC, DuBose JJ, Zhan LX, Hughes JD, Goshima KR, Rhee P, et al. Trends and outcomes of endovascular therapy in the management of civilian vascular injuries. J Vasc Surg. 2014; 60:1297–1307. 1307.e1
13. Desai SS, DuBose JJ, Parham CS, Charlton-Ouw KM, Valdes J, Estrera AL, et al. Outcomes after endovascular repair of arterial trauma. J Vasc Surg. 2014; 60:1309–1314.
14. du Toit DF, Lambrechts AV, Stark H, Warren BL. Long-term results of stent graft treatment of subclavian artery injuries: management of choice for stable patients? J Vasc Surg. 2008; 47:739–743.
15. Matsagkas M, Kouvelos G, Peroulis M, Xanthopoulos D, Bouris V, Arnaoutoglou E. Endovascular repair of blunt axillo-subclavian arterial injuries as the first line treatment. Injury. 2016; 47:1051–1056.
16. Trellopoulos G, Georgiadis GS, Aslanidou EA, Nikolopoulos ES, Pitta X, Papachristodoulou A, et al. Endovascular management of peripheral arterial trauma in patients presenting in hemorrhagic shock. J Cardiovasc Surg (Torino). 2012; 53:495–506.
17. Macedo FI, Sciarretta JD, Salsamendi J, Karmacharya J, Romano A, Namias N. Repair of an acute blunt popliteal artery trauma via endovascular approach. Ann Vasc Surg. 2015; 29:366.e5–366.e10.
18. Hafez HM, Woolgar J, Robbs JV. Lower extremity arterial injury: results of 550 cases and review of risk factors associated with limb loss. J Vasc Surg. 2001; 33:1212–1219.
19. Debakey ME, Simeone FA. Battle injuries of the arteries in world war II: an analysis of 2,471 cases. Ann Surg. 1946; 123:534–579.
20. Dua A, Desai SS, Shah JO, Lasky RE, Charlton-Ouw KM, Azizzadeh A, et al. Outcome predictors of limb salvage in traumatic popliteal artery injury. Ann Vasc Surg. 2014; 28:108–114.
21. Wolma FJ, Larrieu AJ, Alsop GC. Arterial injuries of the legs associated with fractures and dislocations. Am J Surg. 1980; 140:806–809.
22. McArthur CS, Marin ML. Endovascular therapy for the treatment of arterial trauma. Mt Sinai J Med. 2004; 71:4–11.
23. Rocha L, Dalio MB, Joviliano EE, Piccinato CE. Endovascular approach for peripheral arterial injuries. Ann Vasc Surg. 2013; 27:587–593.
24. Kropman RH, Kiela G, Moll FL, de Vries JP. Variations in anatomy of the popliteal artery and its side branches. Vasc Endovascular Surg. 2011; 45:536–540.
25. Klecker RJ, Winalski CS, Aliabadi P, Minas T. The aberrant anterior tibial artery: magnetic resonance appearance, prevalence, and surgical implications. Am J Sports Med. 2008; 36:720–727.
26. Becker GJ, Benenati JF, Zemel G, Sallee DS, Suarez CA, Roeren TK, et al. Percutaneous placement of a balloon-expandable intraluminal graft for life-threatening subclavian arterial hemorrhage. J Vasc Interv Radiol. 1991; 2:225–229.
27. Sciarretta JD, Macedo FI, Otero CA, Figueroa JN, Pizano LR, Namias N. Management of traumatic popliteal vascular injuries in a level I trauma center: a 6-year experience. Int J Surg. 2015; 18:136–141.
28. Hutto JD, Reed AB. Endovascular repair of an acute blunt popliteal artery injury. J Vasc Surg. 2007; 45:188–190.
29. Cervin A, Tjärnström J, Ravn H, Acosta S, Hultgren R, Welander M, et al. Treatment of popliteal aneurysm by open and endovascular surgery: a contemporary study of 592 procedures in Sweden. Eur J Vasc Endovasc Surg. 2015; 50:342–350.
30. Ronchey S, Pecoraro F, Alberti V, Serrao E, Orrico M, Lachat M, et al. Popliteal artery aneurysm repair in the endovascular era: fourteen-years single center experience. Medicine (Baltimore). 2015; 94:e1130.
31. Kim YH, Bae JI, Jeon YS, Kim CW, Jae HJ, Park KB, et al. Korean guidelines for interventional recanalization of lower extremity arteries. Korean J Radiol. 2015; 16:696–722.
32. Chang IS, Chee HK, Park SW, Yun IJ, Hwang JJ, Lee SA, et al. The primary patency and fracture rates of self-expandable nitinol stents placed in the popliteal arteries, especially in the P2 and P3 segments, in Korean patients. Korean J Radiol. 2011; 12:203–209.
33. Antonello M, Frigatti P, Battocchio P, Lepidi S, Cognolato D, Dall'Antonia A, et al. Open repair versus endovascular treatment for asymptomatic popliteal artery aneurysm: results of a prospective randomized study. J Vasc Surg. 2005; 42:185–193.
34. Piffaretti G, Tozzi M, Lomazzi C, Rivolta N, Caronno R, Laganà D, et al. Endovascular treatment for traumatic injuries of the peripheral arteries following blunt trauma. Injury. 2007; 38:1091–1097.
35. Nikanorov A, Smouse HB, Osman K, Bialas M, Shrivastava S, Schwartz LB. Fracture of self-expanding nitinol stents stressed in vitro under simulated intravascular conditions. J Vasc Surg. 2008; 48:435–440.
36. Brandt MM, Kazanjian S, Wahl WL. The utility of endovascular stents in the treatment of blunt arterial injuries. J Trauma. 2001; 51:901–905.
37. Ruffino MA, Rabbia C. Italian Cardiatis Registry Investigators Group. Endovascular repair of peripheral and visceral aneurysms with the Cardiatis multilayer flow modulator: one-year results from the Italian Multicenter Registry. J Endovasc Ther. 2012; 19:599–610.
38. Pieper CC, Meyer C, Rudolph J, Verrel F, Schild HH, Wilhelm KE. Interventional exclusion of iliac artery aneurysms using the flow-diverting multilayer stent. Cardiovasc Intervent Radiol. 2013; 36:917–925.
39. Chalmers RT, Hoballah JJ, Sharp WJ, Kresowik TF, Corson JD. The effect of an intraluminal stent on neointimal hyperplasia at an end-to-side polytetrafluoroethylene graft arterial anastomosis. Am J Surg. 1994; 168:85–90.
40. Chalmers RT, Hoballah JJ, Sharp WJ, Kresowik TF, Corson JD. Effect of an endovascular stent on healing of an end-to-end polytetrafluoroethylene-artery anastomosis in a canine model. Br J Surg. 1994; 81:1443–1447.
41. Henry M, Amor M, Cragg A, Porte JM, Henry I, Amicabile C, et al. Occlusive and aneurysmal peripheral arterial disease: assessment of a stent-graft system. Radiology. 1996; 201:717–724.
42. Resnick S, Chiang A. Transcatheter embolization of a high-flow renal arteriovenous fistula with use of a constrained wallstent to prevent coil migration. J Vasc Interv Radiol. 2006; 17(2 Pt 1):363–367.
43. Yu PT, Rice-Townsend S, Naheedy J, Almodavar H, Mooney DP. Delayed presentation of traumatic infrapopliteal arteriovenous fistula and pseudoaneursym in a 10-year-old boy managed by coil embolization. J Pediatr Surg. 2012; 47:e7–e10.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download