Abstract
Objective
To evaluate the mid-term outcomes of percutaneous radiofrequency ablation (RFA) treatment in patients with small (< 4 cm) renal cell carcinoma (RCC) in Korea.
Materials and Methods
Between 2010 and 2015, 51 patients (40 men and 11 women; median age, 57 years) with biopsyproven 51 RCC were treated using CT-guided RFA. All patients were clinically staged T1aN0M0 prior to RFA. The median tumor size and follow-up period were 2.1 cm (range, 1.0–3.9 cm) and 26 months (4–60 months), respectively. Local tumor progression, distant metastasis, primary and secondary effectiveness rates, and major complication rates were recorded. Estimated glomerular filtration rates (GFRs) between pre-RFA and last follow-up were compared using paired t tests. The 2-year recurrence-free survival rate was calculated using Kaplan-Meier survival analysis.
Results
Of the 51 patients, 2 (3.9%) experienced local tumor progression, and 1 (2.0%) had lymph node metastasis after the first RFA session. Primary and secondary effectiveness rates were 96.1% (49/51) and 100% (1/1), respectively. Only 1 patient experienced a major complication (uretero-pelvic stricture) after the second RFA session for treating a local tumor progression, and the major complication rate was 1.9% (1/52). The median pre-RFA and last follow-up GFRs were 87.1 mL/ min/1.73 m2 (14.2–142.7 mL/min/1.73 m2) and 72.0 mL/min/1.73 m2 (7.2–112.6 mL/min/1.73 m2), respectively (p < 0.0001). The 2-year recurrence-free survival rate was 96.0%.
Percutaneous radiofrequency ablation (RFA) is an accepted minimally invasive treatment for renal cell carcinoma (RCC) in patients who are unable to undergo surgery. Recently, the long-term outcomes of patients treated with RFA were published for treatment of Western patients with RCC (1,2,3,4). However, there has been no study to date on the treatment outcomes of Asian patients with histologically proven T1a RCC using image-guided RFA. Our hypothesis was that RFA treatment leads to good treatment outcomes in Korean patients with small RCCs.
The purpose of this study was to evaluate the mid-term outcomes of CT-guided RFA in the treatment of patients with small (< 4 cm) RCC in Korea.
This retrospective study was approved by our Institutional Review Board. Informed consent of patients was waived.
Between January 2010 and December 2014, 64 patients underwent CT-guided RFA of renal tumors. The inclusion criteria were renal mass biopsy, biopsy-proven RCC, small (< 4 cm) RCC without metastasis, sporadic RCC, renal function tests before and after RFA, and at least 1 month of imaging follow-up. Thirteen patients were excluded due to non-diagnostic tissue (n = 3), hereditary RCC (n = 3), T1b RCC (n = 2), renal metastasis from lung cancer (n = 1), follow-up loss (n = 1), pulmonary metastasis before RFA (n = 1), RCC arising from a transplanted kidney (n = 1), and incomplete RFA session due to severe bleeding (n = 1).
A total of 51 patients (40 men and 11 women; median age, 57 years; age range, 34–80 years) with 51 RCC were finally included in the analyses. The patients all had clinical stage Ia disease with RCCs < 4 cm, no vein thrombosis, and no metastasis. Percutaneous renal mass biopsies were all performed under ultrasonography (n = 47) or CT (n = 4) guidance prior to RFA. RCC size was measured on contrast-enhanced CT or MR images, and the median tumor size was 2.1 cm (range, 1.0–3.9 cm). The RCC subtypes were clear cell (n = 41), papillary (n = 2), chromophobe (n = 2), and unclassified (n = 6).
Radiofrequency ablation was indicated for one or more of the following comorbidities: coexisting cancer, previous partial or radical nephrectomy, chronic kidney disease, chronic liver disease, diabetes mellitus, aortic aneurysm, bleeding tendencies or coagulopathy, and old age. For patient selection, all patient clinical and laboratory findings were reviewed by a multidisciplinary team composed of a urologic oncologist, a medical oncologist, a radiation oncologist, and an interventional radiologist.
Percutaneous RFA was performed by a radiologist with 7 years of experience at the time of the first RFA and 11 years of experience at the time of the last RFA. RFA was guided using a CT scanner (Aquilion, Toshiba Medical Systems Corp., Otawara, Japan).
Conscious sedation was administered to 10 patients who underwent RFA before June 2011. Many patients complained of severe pain, which made it difficult to perform the RFA procedure. After June 2011, general anesthesia was administered to 41 patients. For conscious sedation, midazolam, 1–2 mg (Bukwang Pharmaceutical Co. Ltd., Seoul, Korea), and fentanyl, 50–100 mcg (Hana Pharm Co. Ltd., Seoul, Korea), were intravenously injected.
CT-guided RFA procedures consisted of planning, targeting, monitoring, and surveying phases (5). One or more CT scans were performed during each phase. Each ablation was stopped whenever electrical power first dropped to 0 W (6, 7). The RFA session ended when an interventional radiologist ensured that the renal tumor and at least 5 mm tumor margin were completely ablated on CT images (7).
A Cool-tip RF electrode (Radionics, Inc., Burlington, MA, USA) or Proteus RF electrode (STARmed, Goyang, Korea) was used for RFA. Both electrodes were internally cooled with pump-circulated saline. However, the length of the uninsulated Cool-tip RF electrode was fixed at 2 or 3 cm, whereas that of the uninsulated Proteus RF electrode was controlled every 0.5 cm (range, 0.5–3 cm). A generator (Radionics, Inc., or STARmed) was used to monitor tissue impedance and adjust maximum energy delivery automatically. RFA was performed using a single electrode alone and not with clustered or multiple electrodes. Generator power was maintained at 50 W for the first 30 seconds and gradually increased to 150–180 W thereafter, until electrical power was first dropped to 0 W. During the ablation procedure, tissue resistance ranged from 51 to 70 Ω (mean, 61 Ω).
Patients were positioned prone on the CT table. When a RCC was located in the upper pole, the patient was placed in a left or right anterior oblique position to partially collapse the ipsilateral lung. When a RCC was close to the bowel or ureter, non-invasive or invasive prevention procedures were performed. Non-invasive prevention procedures included patient position change and/or levering electrode. Invasive prevention procedures included hydrodissection and/or ureter catheterization.
All patients underwent unenhanced and contrast-enhanced CT scans prior to RFA. Of the 51 patients, 48 patients were evaluated using unenhanced and contrast-enhanced CT scans after RFA. The remaining 3 patients underwent unenhanced and contrast-enhanced MRI for follow-up, and < 0.5 mmol/kg of contrast material was intravenously injected. The follow-up period after RFA occurred between February 2010 and January 2016 (median, 26 months; range, 4–60 months). All patients underwent CT scans before RF ablation. Pre-RFA and post-RFA CT examinations were performed in 1 of 3 CT scanners (GE LightSpeed VCT, GE Healthcare, Milwaukee, WI, USA; Brilliance 40, Philips Medical Systems, Cleveland, OH, USA; Aquilion, Toshiba Medical Systems Corp.). MRI examination was performed in a 3-tesla MRI scanner (Intera Achieva 3T; Philips Medical Systems, Best, the Netherlands). CT or MRI was performed 1, 6, 12, 18, and 24 months after RFA and every year thereafter.
Renal cell carcinomas were classified as exophytic, endophytic, or mixed tumors according to lesion location. For exophytic tumors, ≥ 75% of tumors projected out from the renal contour. For endophytic tumors, ≤ 25% of tumors projected out from the renal contour. For mixed tumors, 25–75% of tumors projected out from the renal contour.
Duration of ablation, number of electrode repositions, RFA sessions, residual tumor, technical effectiveness, and local tumor progression were recorded. Duration of ablation was defined as the length of time that was needed for RF energy to be delivered in one session. Residual tumor was defined as a focal lesion enhancement detected on the first follow-up CT scan 1 month after RFA. Technical effectiveness was defined when the tumor was successfully treated according to protocol and had no enhancement on the first follow-up images (8). Local tumor progression was defined when a focal enhancement was seen in the lesion on the second follow-up images (8). Primary and secondary effectiveness rates were calculated. The primary effectiveness rate was defined as the percentage of tumors that were successfully eradicated after the initial RFA (8). The secondary effectiveness rate was defined as the percentage of tumors that were successfully eradicated after repeat ablation performed for local tumor progression (8). The numbers of patients with local tumor progression or metastasis on follow-up CT or MR images were recorded. The two-year recurrence-free survival rate was also calculated.
Serum creatinine levels and estimated glomerular filtration rates (GFRs) were checked before and after RFA. GFRs were calculated using the modification of diet in renal disease equation (9). Pre-RFA creatinine levels and GFRs were compared with those at the last follow-up.
The number of CT scans and dose length product (DLP) were recorded from the patient protocols of each RFA CT. The effective dose was calculated for each RFA CT using the method proposed by the European Working Group for Guidelines on Quality Criteria in CT, applying the following relationship: effective dose = DLP × k, where the abdominal k conversion coefficient was 0.015 mSv/mGy·cm (10).
The types (major or minor) of complications and their frequencies were recorded according to the Society of Interventional Radiology Standards of Practice Guidelines (11).
Wilcoxon signed-rank test was used to compare serum creatinine levels measured pre-RFA and those from the last follow up because these data did not follow a Gaussian distribution. Paired t test was used to compare pre-RFA GFRs and those from the last follow up. Kaplan-Meier survival analysis was used to calculate the two-year recurrence-free survival rate. All statistical analyses were performed using commercially available software (PASW Statistics, version 17; SPSS, Inc., Chicago, IL, USA). A p value < 0.05 was considered statistically significant.
Endophytic, mixed, and exophytic RCCs were 54.9% (28/51), 29.4% (15/51), and 15.7% (8/51), respectively (Figs. 1, 2). Hyrodissection was performed in 3 cases and ureter catheterization was also performed in 1 of these cases. All RFA procedures were technically successful, and no tumor was detected 1 month after the procedure by CT or MRI examination (Fig. 1). The median number of electrode repositions was 2 (range, 0–9), and the median RFA duration was 19 minutes (range, 7–57 minutes). Of the 51 patients, 50 underwent 1 RFA session, and 1 underwent 2 sessions.
Local tumor progression was detected in 2 of the 51 patients 7 and 12 months after RFA (Fig. 2). Therefore, the local tumor progression rate was 3.9% (2/51). The sizes of these RCCs were 2.7 cm and 3.2 cm, and both tumors were endophytic. One patient was treated with radical nephrectomy, and the other underwent an additional RFA. Neither patient has experienced any additional recurrence since the second treatment. Primary and secondary effectiveness rates were 96.1% (49/51) and 100% (1/1), respectively. Lymph node metastasis was detected in 1 (2%) patient 7 months after RFA even though there was no local tumor progression. This patient was treated with chemotherapy. The 2 year recurrence-free survival rate was 96.0% (Fig. 3).
The median pre-RFA creatinine level was 0.88 mg/dL, which significantly increased to 1.04 mg/dL at the last follow-up (p < 0.0001) (Table 1). The median pre-RFA GFR was 87.1 mL/min/1.73 m2, which significantly decreased to 72.0 mL/min/1.73 m2 (p < 0.0001) (Table 1).
The median number of CT scans during 1 RFA session was 22 (range, 10–67). The median DLP and effective dose were 1336.1 mGy·cm (range, 463.2–3391.7 mGy·cm) and 20.0 mSv (range, 6.9–50.9 mSv), respectively.
In the 52 RFA sessions, only 1 major complication of uretero-pelvic stricture was detected (1.9%) after the second session RFA to treat local tumor progression (Fig. 2). After the 52 RFA sessions, minor complications were detected in 2 patients (3.9%). Both of these minor complications were peri-renal hematoma, and both spontaneously disappeared.
Our results showed that CT-guided RFA offered low local tumor progression rate, low complication rate and high recurrence-free survival rate in treating patients with a small RCC. Renal function was minimally reduced after RFA, but pre-RFA creatinine levels and GFRs were significantly different from those obtained at the last follow-up. The radiation doses were relatively high due to the large number of CT scans.
Reportedly, long-term recurrence-free survival rates range from 92.3–100% when treating patients with T1a RCCs (1,2,3,4). Zagoria et al. (1) and other groups (2,3,4) have reported that recurrence-free survival rates were 100% and 92.3–95.4% after RFA treatment for T1a RCC, respectively. However, the number of T1a RCCs in the Zagoria study (1) was smaller than those of the other groups (2,3,4). Two-year recurrence-free survival rate is a mid-term outcome, and is not equivalent to the five-year recurrence-free survival rate. However, recurrent RCCs are usually detected post-operatively within 2 years (12). In our study, 2 cases of local tumor progression and 1 metastasis were detected 7–12 months after RFA. Thus, the long-term recurrence-free survival rate is likely to be similar to our mid-term survival rate.
Renal cell carcinoma size and location may also influence local tumor progression after percutaneous RFA. Large (> 3 cm) or endophytic RCC is more likely to progress locally or lead to reduced recurrence-free survival rates (1, 2, 13). The median tumor size (2.1 cm) of our study was smaller than those of previously reported studies (1,2,3,4). However, the percentage of endophytic tumors in our study was greater than those of previously reported studies (2, 13). Guiding modality is a factor that influences treatment outcomes. CT guiding is better than ultrasonography (US) guiding for RCC treatment (14). Air bubbles that are created during RFA may prevent precise assessment of tumor extent or tumor margin that needs to be ablated. Therefore, US-guided RFA is more likely to result in local tumor progression than CT-guided RFA (14).
We switched from conscious sedation to general anesthesia to control pain during our study period. In our study, local tumor progression was detected in 2 patients only after RFA treatment was performed with conscious sedation. No local tumor progression was detected after RFA was performed with general anesthesia. RFA is more painful than cryoablation (15). In some our study cases, pain was not as controlled with conscious sedation alone. This led to early RFA procedures and consequently, incomplete ablation of the tumor margin and local tumor progression. However, further investigation is necessary to assess how general anesthesia influences RFA treatment outcomes.
Percutaneous RFA may lead to excellent treatment outcomes similar to those of partial nephrectomy (4, 16). RFA outcomes are observed for patients who are poor surgical candidates (1,2,3,4, 16). Therefore, RFA might potentially be an alternative treatment in healthy patients who do not want surgery. Moreover, RFA tends to preserve renal function better than partial nephrectomy (4, 16). However, randomized controlled trials are necessary to demonstrate the lack of difference in oncologic outcomes between RFA and partial nephrectomy.
Many studies have reported no differences in renal function before and after RFA (1,2,3). However, we cannot avoid renal function reduction because at least 5 mm tumor margin should be ablated (4, 17). Park et al. (17) have demonstrated that the tumor margin volume is larger than that of the endophytic RCC. As a result, substantial loss of normal renal tissue is essential to prevent local tumor progression. Subsequently, renal function is minimally, but significantly, reduced when treating a single RCC with RFA. Increasing number of RCCs to be ablated may exacerbate the renal function reduction, which has been shown when treating recurrent RCC in patients with von-Hippel-Lindau disease (18,19,20). RCC size and location may also influence renal function change. A patient with large and endophytic RCC is more likely to have reduced renal function than a patient with small and exophytic RCC (17).
Reportedly, major complication rates following RFA are very low (21). These complications include bowel perforation (22), ureter stricture (19), and massive bleeding (23). Our study detected 1 major complication after the second RFA session to treat local tumor progression in 1 patient i.e., uretero-pelvic stricture resulting from heat injury delivered during the RFA procedures. Pyeloperfusion was not performed in this case. Prior to RFA, CT or MR images should be carefully evaluated to determine the necessity of prevention methods, including position change, levering electrode, hydrodissection, and pyeloperfusion (21).
The radiation dose incurred during ablation was relatively higher than that of a single-phase contrast-enhanced abdominopelvic CT scan (5, 24). The median effective dose was 35.3 mSv during CT-guided RFA of liver tumors (25). However, our study showed that the median effective dose was 20.2 mSv during CT-guided RFA of RCC. Many studies that examine long-term outcomes of patients with RCCs treated with RFA have not reported their radiation dose (1,2,3,4). Radiation dose is a serious problem because CT scans are commonly used before RFA, during RFA procedures, and during post-RFA follow-up (24). Therefore, the range, number, current, and voltage of RFA CT scans should be minimized as much as possible.
Our study had some limitations. First, it was conducted in a retrospective manner. Therefore, selection bias cannot be avoided for the study population. Second, the median follow-up period was only 26 months. Our study did not examine the long-term outcomes of patients with T1a RCCs treated using RFA. Third, RFA and partial nephrectomy were not compared in terms of treatment outcomes based on a propensity score analysis. Partial nephrectomy has been increasingly performed for treating patients with T1a RCCs. Some studies reported that RFA could provide comparable 5-year oncologic outcomes to partial nephrectomy and better preservation of renal function. Comparing RFA and other treatments such as cryoablation, microwave, or other thermal ablations will be necessary to determine optimum treatment of RCCs.
In conclusion, CT-guided RFA can offer Korean patients with small RCCs excellent mid-term outcomes, including high primary effectiveness rate, low major complication rate, and high recurrence-free survival rate. However, this treatment modality may lead to reduced renal function and high radiation dose. Therefore, great care should be taken not to further reduce renal function in patients with marginal renal function. CT protocols should be re-evaluated to minimize radiation dose to patients during RFA treatment and follow up.
Figures and Tables
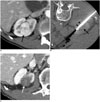 | Fig. 167-year-old man with clear cell RCC.
A. Pre-RFA contrast-enhanced CT image shows 3.1 cm endophytic RCC (arrow) in right kidney. Histologic diagnosis was confirmed clear cell RCC by US-guided biopsy. Pre-RFA creatinine and GFR were 0.98 mg/dL and 76.3 mL/min/1.73 m2, respectively. B. CT-guided RFA was performed with patient prone on CT table. CT image shows electrode (arrowheads) placed within tumor (arrow). C. 33-month post-RFA contrast-enhanced CT image shows complete tumor ablation (arrow) and no local tumor progression. Patient's creatinine increased to 1.0 mg/dL, and GFR decreased to 74.1 mL/min/1.73 m2. GFR = glomerular filtration rate, RCC = renal cell carcinoma, RFA = radiofrequency ablation, US = ultrasonography
|
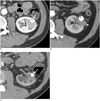 | Fig. 248-year-old man with clear cell RCC.
A. Pre-RFA contrast-enhanced CT image shows 2.7 cm endophytic RCC (arrow) in left kidney. Histologic diagnosis was confirmed clear cell RCC with US-guided biopsy. Pre-RFA creatinine and GFR were 0.65 mg/dL and 131.1 mL/min/1.73 m2, respectively. B. Twelve-month contrast-enhanced CT image after first RFA shows nodular enhancement (arrowhead) at lateral aspect of tumor, suggesting local tumor progression. Second RFA was performed to treat recurrent RCC. C. Forty-month contrast-enhanced CT image after second RFA shows complete ablation of tumor (arrow). However, left kidney becomes atrophic because of uretero-pelvic stricture (arrowhead). Patient's creatinine increased to 1.11 mg/dL, and GFR decreased to 69.6 mL/min/1.73 m2. GFR = glomerular filtration rate, RCC = renal cell carcinoma, RFA = radiofrequency ablation, US = ultrasonography
|
 | Fig. 3Kaplan-Meier survival analysis.This Kaplan-Meier survival curve illustrates excellent recurrence-free survival rate for patients with T1a RCC undergoing percutaneous RFA. Two-year recurrence-free survival rate is 96.0%. RCC = renal cell carcinoma, RFA = radiofrequency ablation
|
References
1. Zagoria RJ, Pettus JA, Rogers M, Werle DM, Childs D, Leyendecker JR. Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. Urology. 2011; 77:1393–1397.
2. Psutka SP, Feldman AS, McDougal WS, McGovern FJ, Mueller P, Gervais DA. Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol. 2013; 63:486–492.
3. Lorber G, Glamore M, Doshi M, Jorda M, Morillo-Burgos G, Leveillee RJ. Long-term oncologic outcomes following radiofrequency ablation with real-time temperature monitoring for T1a renal cell cancer. Urol Oncol. 2014; 32:1017–1023.
4. Chang X, Liu T, Zhang F, Ji C, Zhao X, Wang W, et al. Radiofrequency ablation versus partial nephrectomy for clinical T1a renal-cell carcinoma: long-term clinical and oncologic outcomes based on a propensity score analysis. J Endourol. 2015; 29:518–525.
5. Park BK, Morrison PR, Tatli S, Govindarajulu U, Tuncali K, Judy P, et al. Estimated effective dose of CT-guided percutaneous cryoablation of liver tumors. Eur J Radiol. 2012; 81:1702–1706.
6. Park BK, Kim CK, Lee HM. Image-guided radiofrequency ablation of Bosniak category III or IV cystic renal tumors: initial clinical experience. Eur Radiol. 2008; 18:1519–1525.
7. Park JJ, Park BK, Park SY, Kim CK. Percutaneous radiofrequency ablation of sporadic Bosniak III or IV lesions: treatment techniques and short-term outcomes. J Vasc Interv Radiol. 2015; 26:46–54.
8. Goldberg SN, Grassi CJ, Cardella JF, Charboneau JW, Dodd GD 3rd, Dupuy DE, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009; 20:7 Suppl. S377–S390.
9. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999; 130:461–447.
10. Huda W, Ogden KM, Khorasani MR. Converting dose-length product to effective dose at CT. Radiology. 2008; 248:995–1003.
11. Cardella JF, Kundu S, Miller DL, Millward SF, Sacks D;. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2009; 20:7 Suppl. S189–S191.
12. Chae EJ, Kim JK, Kim SH, Bae SJ, Cho KS. Renal cell carcinoma: analysis of postoperative recurrence patterns. Radiology. 2005; 234:189–196.
13. Gervais DA, McGovern FJ, Arellano RS, McDougal WS, Mueller PR. Radiofrequency ablation of renal cell carcinoma: part 1, indications, results, and role in patient management over a 6-year period and ablation of 100 tumors. AJR Am J Roentgenol. 2005; 185:64–71.
14. Park BK, Kim CK, Choi HY, Lee HM, Jeon SS, Seo SI, et al. Limitation for performing ultrasound-guided radiofrequency ablation of small renal masses. Eur J Radiol. 2010; 75:248–252.
15. Allaf ME, Varkarakis IM, Bhayani SB, Inagaki T, Kavoussi LR, Solomon SB. Pain control requirements for percutaneous ablation of renal tumors: cryoablation versus radiofrequency ablation--initial observations. Radiology. 2005; 237:366–370.
16. Sung HH, Park BK, Kim CK, Choi HY, Lee HM. Comparison of percutaneous radiofrequency ablation and open partial nephrectomy for the treatment of size- and location-matched renal masses. Int J Hyperthermia. 2012; 28:227–234.
17. Park SY, Park BK, Kim CK. Thermal ablation in renal cell carcinoma: what affects renal function? Int J Hyperthermia. 2012; 28:729–734.
18. Park BK, Kim CK. Percutaneous radio frequency ablation of renal tumors in patients with von Hippel-Lindau disease: preliminary results. J Urol. 2010; 183:1703–1707.
19. Park SY, Park BK, Kim CK, Lee HM, Jeon SS, Seo SI, et al. Percutaneous radiofrequency ablation of renal cell carcinomas in patients with von Hippel Lindau disease previously undergoing a radical nephrectomy or repeated nephron-sparing surgery. Acta Radiol. 2011; 52:680–685.
20. Park BK, Kim CK, Park SY, Shen SH. Percutaneous radiofrequency ablation of renal cell carcinomas in patients with von Hippel Lindau disease: indications, techniques, complications, and outcomes. Acta Radiol. 2013; 54:418–427.
21. Park BK, Kim CK. Complications of image-guided radiofrequency ablation of renal cell carcinoma: causes, imaging features and prevention methods. Eur Radiol. 2009; 19:2180–2190.
22. Meloni MF, Goldberg SN, Moser V, Piazza G, Livraghi T. Colonic perforation and abscess following radiofrequency ablation treatment of hepatoma. Eur J Ultrasound. 2002; 15:73–76.
23. Park BK, Kim CK, Moo HL. Arteriovenous fistula after radiofrequency ablation of a renal tumor located within the renal sinus. J Vasc Interv Radiol. 2007; 18:1183–1185.
24. Eisenberg JD, Gervais DA, Singh S, Kalra MK, Sabir SH, Paul AB, et al. Radiation exposure from CT-guided ablation of renal masses: effects on life expectancy. AJR Am J Roentgenol. 2015; 204:335–342.
25. Tsalafoutas IA, Tsapaki V, Triantopoulou C, Gorantonaki A, Papailiou J. CT-guided interventional procedures without CT fluoroscopy assistance: patient effective dose and absorbed dose considerations. AJR Am J Roentgenol. 2007; 188:1479–1484.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download