Abstract
Objective
To evaluate the feasibility of angled cool-tip electrode for radiofrequency ablation of small superficial subcapsular liver tumors abutting abdominal wall, in order to traverse normal liver parenchyma, and thereby, obtain favorable configuration of ablation margin.
Materials and Methods
In this study, we retrospectively analyzed 15 small superficial subcapsular liver tumors abutting abdominal wall in 15 patients, treated with radiofrequency ablation from March 2013 to June 2015 using a cool-tip electrode manually modified to create 25–35° angle at the junction between exposed and insulated segments. The tumors were hepatocellular carcinoma (n = 13) and metastases (n = 2: cholangiocellular carcinoma and rectosigmoid cancer), with maximum diameter of 10–26 mm (mean, 15.68 ± 5.29 mm). Under ultrasonographic guidance, the electrode tip was advanced to the depth of the tumors' epicenter about 1 cm from the margin. The tip was re-directed to penetrate the tumor for radiofrequency ablation. Minimal ablation margin was measured at immediate post-treatment CT. Radiological images and medical records were evaluated for success rate, length of minimal ablation margin and complications.
Results
Technical success rate of obtaining complete necrosis of the tumors was 100%, with no procedure-related complication. Minimal ablation margin ranged from 3–12 mm (mean, 7.07 ± 2.23 mm). CT/MRI follow-up at 21–1022 days (mean, 519.47 ± 304.51 days) revealed no local recurrence, but distant recurrence in 9 patients.
Radiofrequency (RF) ablation is an important therapeutic tool for the treatment of primary and secondary liver malignancies (1234). Complete necrosis is achieved in over 90% of cases of small hepatocellular carcinomas ≤ 3 cm in size (45). Results of treatment of very-early and early hepatocellular carcinomas is reportedly comparable to surgery (67). Various complications related to RF ablation of liver tumor have been reported, but the procedure is considered as relatively safe with overall complication rate of 7.2–12% (891011). When the tumor is located at high-risk locations in close proximity to the large vessel, bile duct, extra-hepatic organ or the liver capsule, the incidence of complication may increase. Subsequently, the procedure needs to be performed cautiously or in conjunction with additional special techniques (121314).
Radiofrequency ablation has the disadvantage that obtaining adequate length of ablation margin in all directions may be difficult for index tumor located at the superficial subcapsular region of the liver. In addition, reported incidence of electrode tract seeding is reportedly as high as 12.5% in patients with subcapsular tumors (15). Although subsequent reports suggest much lower incidence of seeding (891011161718192021), RF ablation is generally not a preferred procedure for subcapsular tumors (141516). Subsequently, it was recommended to avoid direct insertion of the electrode into the tumor without traversing normal parenchyma (22). A technique of ablation of subcapsular tumor without direct insertion of the antenna during microwave ablation has also been reported (23).
To obtain sufficient length of ablation margin in all directions and to traverse non-tumorous liver parenchyma during RF ablation, we modified the cool-tip electrode so that it is angled at the junction of insulated and exposed segment. The angled cool-tip electrode was used for RF ablation of small subcapsular tumors abutting the abdominal wall. In this study, we reported the feasibility and results of using the angled cool-tip electrode for RF ablation of superficially located small subcapsular tumors in liver abutting the abdominal wall.
This retrospective study was approved by the Institutional Review Board, and written informed consent for the procedure was obtained from all patients. Requirement of informed consent prior to inclusion was waived due to the retrospective nature of the study. All procedures were performed in accordance with the guidelines of the institution.
From March 2013 to June 2015, among 342 RF ablations for primary and secondary liver malignancies, angled cool-tip electrode was used for RF ablation of 15 superficial subcapsular tumors of 3 cm or smaller in the longest dimension in 15 patients, and was retrospectively evaluated. The patients were 12 men and 3 women with ages ranging from 37 to 69 years (mean, 52.60 ± 8.78 years).
The index tumors were hepatocellular carcinoma (n = 13), metastasis from rectosigmoid cancer (n = 1) and metastasis from cholangiocarcinoma (n = 1). All tumors were newly developed lesions, superficially located, abutting the liver capsule in the abdominal wall side, diagnosed by clinical findings and dynamic CT and/or MRI during follow-up, with the diameter in the longest dimension measuring 10–26 mm (mean, 15.68 ± 5.29 mm). Of the 13 patients with hepatocellular carcinoma, all patients had underlying liver cirrhosis, related to B-viral hepatitis in 10, C-viral hepatitis in 1 and alcoholic cirrhosis in 2 patients. The hepatocellular carcinomas were diagnosed by typical image findings of enhancement on arterial phase with washout on portal or delayed phase (n = 13), and combined increased tumor markers (alpha-fetoprotein [α-FP] > 400, α-FP-L3% > 10, or proteins induced by vitamin K absence or antagonist-II [PIVKA-II] > 40) (n = 9) (2425). The metastasis from cholangiocellular carcinoma was diagnosed by increased size of the tumor on 2 month follow-up liver MRI with increased carcinoembryonic antigen (CEA) level, and the metastasis from rectosigmoid cancer was diagnosed by CT and MRI findings consistent with metastasis and increasing CEA level on follow-up. Positron emission tomography was not performed in patients with metastasis. In all patients, histological diagnosis of the tumor was not performed.
The angled cool-tip electrode was used for RF ablation in any primary or secondary liver tumors that was single in number, with diameter of 3 cm or smaller in the longest dimension in both the axial and the coronal images on liver dynamic CT and/or MRI, and located at superficial supcapsular region of the liver abutting the abdominal wall. Exclusion criteria included any tumor abutting critical organ such as bowel or gall bladder, uncooperative patient, and uncontrolled bleeding tendency. Characteristics of the patients and the tumors were summarized in the Table 1.
In all cases, the 17 G cool-tip electrode of 15 cm length with 3 cm exposed tip (Covidien, Mansfield, MA, USA) was used to prepare the angled electrode. In all cases, the angle was made toward the opposite direction where the tubes and the electrical wire was attached. Using a sterile needle holder, the electrode was angled manually to an angle of 25–35° by adding 4–5 small bends within 5–6 mm length of the electrode, centered at the junction of insulated and exposed segment of the electrode (Fig. 1).
Pre-RF ablation ultrasonography evaluation was performed to determine the skin access site. An imaginary target point within the normal liver parenchyma at the depth of the epicenter of the index tumor and at about 1 cm apart from the tumor margin was selected. From this target point, an imaginary line with about 30° angle from the skin of the patient toward the farther side from the index tumor was drawn. The point where the imaginary line crossed the patient's skin was determined as the skin access site of the angled cool-tip electrode. After sterile preparation of the patient's skin, the pre-determined access site was anesthetized using 1% lidocaine hydrochloride. By holding the proximal, non-angled segment of the electrode more vertically to the patient's skin, the angled segment of the cool-tip electrode was inserted through the predetermined access site at about 30° to the patient's skin. Under ultrasonography guide, the tip of the angled cool-tip electrode was advanced along the imaginary line, toward the imaginary target point. After the tip of the angled cool-tip electrode reached the imaginary target point, the proximal non-angled segment of the electrode was depressed toward the patient's skin to lift up and direct the tip of the angled segment of the electrode toward the index tumors' epicenter. The cool-tip electrode was further advanced to penetrate the index tumor until adequate position was obtained (Fig. 2). RF ablation was performed during continuous circulation of 0–5°C normal saline for cooling the electrode, for a minimum of 12 minute. To obtain at least 1 cm of ablation margin in all directions possible, ablation time was 12 minute when the tumor was ≤ 1 cm in the longest diameter. When the diameter of the index tumor was > 1 cm, ablation time was increased to enlarge the diameter of ablation. According to the operator's discretion coupled with ultrasonographic monitoring of hyper-echogenicity, 3 to 6 minute ablation time was added for each 0.5 cm increase in the longest diameter of the index tumor. When the angled electrode was repositioned during the procedure or when a second angled electrode was used, another 12 minutes of ablation was performed. After the ablation procedure, needle tract coagulation was performed in all patients. All procedures were performed under conscious sedation, using fentanylcitrate and midazolam-hydrochloride. No additional procedure was performed to cool the abdominal wall during the RF ablation procedure.
Post-treatment CT was performed within 1 day after the procedure. Follow-up CT/MRI was performed 1–3 months after the treatment, and at 3 month intervals thereafter. Radiological images and medical records were retrospectively evaluated for success rate, minimal ablation margin and complications.
A superficial subcapsular tumor was defined as the index tumor abutting the liver capsule on the abdominal wall side.
Technical success was defined as complete coagulative necrosis of the index tumor using the angled cool-tip electrode.
Minimal ablation margin was defined as the minimum length of the region ablated beyond the border of the index tumor in axial and coronal images at immediate post-treatment CT.
Mean values were provided as mean ± standard deviation.
All treatments were performed in one session, with number of energy applications ranging from 1–3 (mean, 1.27 ± 0.59), and sum of ablation time ranging from 12–30 minutes (mean, 14.60 ± 5.32 minutes). Additional angled cool-tip electrode insertion was performed in 1 patient (patient 7 on Table 1). Technical success of obtaining complete necrosis of the index tumors was 100%. There was no immediate procedure-related complication.
Minimal ablation margin at immediate post-treatment CT ranged from 3–12 mm (mean, 7.07 ± 2.23 mm).
Final CT/MRI performed during follow-up at 21–1022 days (mean, 519.47 ± 304.51 days) revealed no local recurrence and no electrode tract seeding, but distant recurrence was noted in 9 patients: 8 hepatocellular carcinomas and 1 metastasis from rectosigmoid carcinoma. Among the 8 patients with recurrent hepatocellular carcinomas, multiple sessions of transarterial chemoembolization were performed in 4 patients. The remaining 4 patients expired due to progression of recurrent hepatocelluar carcinoma despite living donor liver transplantation in 1 patient and transarterial chemoembolization in 3 patients for recurrent tumors.
Insufficient ablation margin is a significant risk factor of local tumor recurrence after RF ablation of a liver malignancy (13). When the index tumor is located at high-risk locations, aggressive ablation to obtain sufficient ablation margin may be difficult, resulting in increased complications and local tumor recurrence (12). Subsequently, the procedure needs to be performed cautiously or in conjunction with additional special techniques (14).
For index tumor located at superficial subcapsular region of the liver abutting the abdominal wall, the multi-tined expandable RF ablation electrode has the disadvantage that one or more of the tines may penetrate the liver capsule, resulting in injury of the abdominal wall during ablation. The cool-tip electrode has the disadvantage that the junction of insulated and exposed segment of the electrode cannot be detected by ultrasonography. Subsequently, the electrode may be positioned too deep within the liver parenchyma or may straddle the liver parenchyma and the abdominal wall. Moreover, even when the junction is placed exactly at the capsule of the liver, the ablation margin will be deviated toward the direction of electrode insertion, resulting in insufficient ablation margin in the proximal side.
The angled cool-tip electrode has the advantage that the electrode can be aligned in a symmetric configuration with respect to the index tumor and the liver capsule. In addition, the proximal end of the exposed segment of the electrode, which is the angle, can be detected by ultrasonography. As the angle is detectable, the index tumor can be centered within the exposed segment of the electrode. Subsequently, more favorable, symmetric ablation margin can be expected (Fig. 3). Minimal ablation margin of 5 mm is recommended in RF ablation of colorectal metastasis and 3 mm in hepatocellular carcinoma (222627). In using the angled cool-tip electrode for RF ablation of a superficial subcapsular tumor, a mean ablation margin of 7.07 ± 2.23 mm could be obtained without significant complication during mean follow up of 349.73 days.
Electrode tract seeding is another major delayed complication that can result after RF ablation of a subcapsular tumor as it results in distant metastasis, especially when performed as a bridge therapy for liver transplantation. The risk factors influencing electrode tract seeding include poorly differentiated tumor, high α-FP value in hepatocellular carcinoma, subcapsular location, tumor biopsy prior to RF ablation, numbers of sessions of treatment, numbers of electrode placement, tumor > 3 cm, direct subcapsular insertion of electrode, and intra-tumoral pressure increase (15161718). Decreasing the number of electrode insertion and repositioning, sufficient length of electrode tract, coagulation of electrode tract, and laparoscopic treatment of exophytic or subcapsular lesions are suggested to minimize the incidence of seeding (1117).
During RF ablation of a subcapsular tumor, it is difficult to acquire sufficient length of electrode tract during targeting and coagulation of the tract after the procedure. To avoid electrode tract seeding, direct penetration of the tumor without traversing non-tumorous liver parenchyma should be avoided (22), and laparoscopic RF ablation is recommended (1117). The alternative no-touch wedge ablation technique is reported for microwave ablation of subcapsular tumors in liver. The technique consists of placing the antenna in multiple sites in oblique directions tangential to the index tumor, without directly entering the tumor. The summated ablation zones are required to encompass the entire subcapsular tumor. But the technique has the disadvantage that some learning curve may be required as multiple ablation zones have to be designed to encompass the entire tumor. Occasionally, design of the ablation zones can be limited by intervening ribs or adjacent heat sensitive organs according to the tumor location. Requirement of multiple probe insertions with a mean of 5.6 insertions is another disadvantage of the technique (23). The technique of inducing artificial ascites or CT-guided targeting from distant access site to traverse sufficient length of liver parenchyma may also be used for targeting the superficial subcapsular tumor. But inducing artificial ascites requires additional time and procedure of catheter insertion, and may not be always successful, especially in patients who had undergone prior surgery. CT-guided targeting from distant access site has the disadvantage of radiation exposure and considerable amount of additional time may be required as the electrode has to pass through a lengthy tract, which may not be always easy.
The angled cool-tip electrode prevents direct penetration of the superficial subcapsular tumor, increases the length of electrode tract within non-tumorous liver parenchyma and ablates the electrode tract after ablation. Although the optimum length of electrode tract to minimize seeding is unknown, electrode tract seeding was not detected after RF ablation of subcapsular tumors using the angled cool-tip electrode during a mean follow-up of 349.73 days.
In preparing the angled cool-tip electrode, multiple small bends were made in as short length of the electrode as possible at the junction of insulated and exposed segment to avoid unnecessary exfoliation of the insulation. In addition, multiple small bends were added to angle the electrode rather than a single large bend to avoid fracture of the electrode while adjusting the tip of the electrode toward the epicenter of the tumor. In all our cases, no significant change in the angle of the electrode could be perceived after the procedure. Also, during test perfusion of the perfusate prior to the procedure, there was no leakage of the perfusate. Compared to using non-angled cool-tip electrode, there was no change in the total amount of perfusate used during the procedure and 1200 to 1500 mL of perfusate was used during 12 minute ablation.
We recommend bending the electrode contralateral to the attachment of tubes for perfusate, as in the transjugular intrahepatic portosystemic shunt needle (Fig. 1). Although an experienced interventional radiologist skilled in ultrasonography may perform the targeting without difficulty, bending the exposed electrode tip in a predictable direction would further ease the procedure. In addition, the weight of the tubes and the cable may rotate the electrode during ablation. In our experience, bending the electrode contralateral to the tubes could minimize the effect of weight of the tubes in most cases. Lastly, as the critical steps of the procedure are performed under ultrasonography guidance, high resolution ultrasonography equipment is recommended during targeting and monitoring.
The limitations in the study include its retrospective nature, small number of cases that were enrolled from a single institution, only small tumors included in the study, lack of histological confirmation of the tumor, and limited duration of clinical and imaging follow-up. Subsequently, the true recurrence rate and clinical significance of the technique are not yet known. Long-term follow-up in larger series of patients is required.
In conclusion, using an angled cool-tip electrode for RF ablation of superficial subcapsular tumor may be a feasible technique for adequate ablation margin and lowered incidence of complications. A larger prospective study is needed for a more definitive evaluation of the technique.
Figures and Tables
Fig. 1
Image of angled cool-tip electrode.
Angled cool-tip electrode is prepared manually by applying 4 to 5, multiple small bends at junction of insulated segment and exposed segment of cooltip electrode to angle of 25–35° using sterile needle holder.

Fig. 2
Images of 49-year-old female with hepatocellular carcinoma during follow-up after two sessions of transarterial chemoembolization and RF ablation in other segment of liver.
Dynamic liver MRI reveals recurrent hepatocellular carcinoma (white arrows) with early enhancement on arterial phase image (A) and high signal intensity on T2 weighted image (B) at superficial subcapsular region of segment 2. Sonography (C) obtained during RF ablation of hepatocellular carcinoma. Angled segment of cool-tip electrode (arrowheads) is inserted and advanced at approximately 45° to skin of access site and advanced toward capsule of liver apart from index tumor (arrows). Electrode (arrowhead) is penetrating liver capsule at about 1–2 cm apart from index tumor (arrows) (D). On entering liver parenchyma to about depth of index tumors' epicenter, tip of angled electrode (arrows) is directed toward index tumors' epicenter (E). Electrode is advanced to penetrate index tumor (arrows) until adequate position is obtained, and RF ablation is performed (F). Follow-up CT images performed immediately after (G) and 33 days after (H) RF ablation procedure reveals complete necrosis of tumor with sufficient ablation margin. RF = radiofrequency
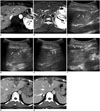
Fig. 3
Diagram of difference between straight and angled cool-tip electrode.
Junction of insulated and exposed segment of cool-tip electrode cannot be detected by ultrasonography. Even if junction is placed exactly at capsule of liver, ablation margin maybe insufficient in proximal electrode side (arrow) (A). When angled cool-tip electrode is used, angle can be detected on ultrasonography, and index tumor can be centered within exposed segment of electrode. Ablation margin will have more symmetric configuration around index tumor (B).
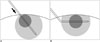
Table 1
Characteristics of Patients and Tumors in Which RFA Was Performed Using Angled Cool-Tip Electrode

CCC = metastasis from cholangiocellular carcinoma, CEA = carcinoembryonic antigen, HAIC = hepatic artery infusion chemotherapy, HCC = hepatocellular carcinoma, PIVKA-II = proteins induced by vitamin K absence or antagonist-II, RFA = radiofrequency ablation, RSC = metastasis from rectosigmoid cancer, TACE = transcatheter arterial chemoembolization, α-FP = alpha-fetoprotein
References
1. Shiina S, Tateishi R, Arano T, Uchino K, Enooku K, Nakagawa H, et al. Radiofrequency ablation for hepatocellular carcinoma: 10-year outcome and prognostic factors. Am J Gastroenterol. 2012; 107:569–577. quiz 578.
2. Livraghi T, Goldberg SN, Lazzaroni S, Meloni F, Solbiati L, Gazelle GS. Small hepatocellular carcinoma: treatment with radio-frequency ablation versus ethanol injection. Radiology. 1999; 210:655–661.
3. Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001; 221:159–166.
4. Yoon JH, Lee JM, Hwang EJ, Hwang IP, Baek J, Han JK, et al. Monopolar radiofrequency ablation using a dual-switching system and a separable clustered electrode: evaluation of the in vivo efficiency. Korean J Radiol. 2014; 15:235–244.
5. Rossi S, Buscarini E, Garbagnati F, Di Stasi M, Quaretti P, Rago M, et al. Percutaneous treatment of small hepatic tumors by an expandable RF needle electrode. AJR Am J Roentgenol. 1998; 170:1015–1022.
6. Pompili M, Saviano A, de Matthaeis N, Cucchetti A, Ardito F, Federico B, et al. Long-term effectiveness of resection and radiofrequency ablation for single hepatocellular carcinoma ≤3 cm. Results of a multicenter Italian survey. J Hepatol. 2013; 59:89–97.
7. Cho YK, Kim JK, Kim WT, Chung JW. Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology. 2010; 51:1284–1290.
8. de Baère T, Risse O, Kuoch V, Dromain C, Sengel C, Smayra T, et al. Adverse events during radiofrequency treatment of 582 hepatic tumors. AJR Am J Roentgenol. 2003; 181:695–700.
9. Mulier S, Mulier P, Ni Y, Miao Y, Dupas B, Marchal G, et al. Complications of radiofrequency coagulation of liver tumours. Br J Surg. 2002; 89:1206–1222.
10. Livraghi T, Solbiati L, Meloni MF, Gazelle GS, Halpern EF, Goldberg SN. Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology. 2003; 226:441–451.
11. Rhim H, Yoon KH, Lee JM, Cho Y, Cho JS, Kim SH, et al. Major complications after radio-frequency thermal ablation of hepatic tumors: spectrum of imaging findings. Radiographics. 2003; 23:123–134. discussion 134-136.
12. Teratani T, Yoshida H, Shiina S, Obi S, Sato S, Tateishi R, et al. Radiofrequency ablation for hepatocellular carcinoma in so-called high-risk locations. Hepatology. 2006; 43:1101–1108.
13. Kim YS, Rhim H, Cho OK, Koh BH, Kim Y. Intrahepatic recurrence after percutaneous radiofrequency ablation of hepatocellular carcinoma: analysis of the pattern and risk factors. Eur J Radiol. 2006; 59:432–441.
14. Yang W, Yan K, Wu GX, Wu W, Fu Y, Lee JC, et al. Radiofrequency ablation of hepatocellular carcinoma in difficult locations: strategies and long-term outcomes. World J Gastroenterol. 2015; 21:1554–1566.
15. Llovet JM, Vilana R, Brú C, Bianchi L, Salmeron JM, Boix L, et al. Increased risk of tumor seeding after percutaneous radiofrequency ablation for single hepatocellular carcinoma. Hepatology. 2001; 33:1124–1129.
16. Shirai K, Tamai H, Shingaki N, Mori Y, Moribata K, Enomoto S, et al. Clinical features and risk factors of extrahepatic seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Hepatol Res. 2011; 41:738–745.
17. Jaskolka JD, Asch MR, Kachura JR, Ho CS, Ossip M, Wong F, et al. Needle tract seeding after radiofrequency ablation of hepatic tumors. J Vasc Interv Radiol. 2005; 16:485–491.
18. Imamura J, Tateishi R, Shiina S, Goto E, Sato T, Ohki T, et al. Neoplastic seeding after radiofrequency ablation for hepatocellular carcinoma. Am J Gastroenterol. 2008; 103:3057–3062.
19. Livraghi T, Lazzaroni S, Meloni F, Solbiati L. Risk of tumour seeding after percutaneous radiofrequency ablation for hepatocellular carcinoma. Br J Surg. 2005; 92:856–858.
20. Sartori S, Tombesi P, Macario F, Nielsen I, Tassinari D, Catellani M, et al. Subcapsular liver tumors treated with percutaneous radiofrequency ablation: a prospective comparison with nonsubcapsular liver tumors for safety and effectiveness. Radiology. 2008; 248:670–679.
21. Poon RT, Ng KK, Lam CM, Ai V, Yuen J, Fan ST. Radiofrequency ablation for subcapsular hepatocellular carcinoma. Ann Surg Oncol. 2004; 11:281–289.
22. Crocetti L, de Baere T, Lencioni R. Quality improvement guidelines for radiofrequency ablation of liver tumours. Cardiovasc Intervent Radiol. 2010; 33:11–17.
23. Patel PA, Ingram L, Wilson ID, Breen DJ. No-touch wedge ablation technique of microwave ablation for the treatment of subcapsular tumors in the liver. J Vasc Interv Radiol. 2013; 24:1257–1262.
24. Korean Liver Cancer Study Group (KLCSG). National Cancer Center, Korea (NCC). 2014 Korean Liver Cancer Study Group-National Cancer Center Korea practice guideline for the management of hepatocellular carcinoma. Korean J Radiol. 2015; 16:465–522.
25. Yoon JH, Park JW, Lee JM. Noninvasive diagnosis of hepatocellular carcinoma: elaboration on Korean Liver Cancer Study Group-National Cancer Center Korea practice guidelines compared with other guidelines and remaining issues. Korean J Radiol. 2016; 17:7–24.
26. Wang X, Sofocleous CT, Erinjeri JP, Petre EN, Gonen M, Do KG, et al. Margin size is an independent predictor of local tumor progression after ablation of colon cancer liver metastases. Cardiovasc Intervent Radiol. 2013; 36:166–175.
27. Kim YS, Lee WJ, Rhim H, Lim HK, Choi D, Lee JY. The minimal ablative margin of radiofrequency ablation of hepatocellular carcinoma (> 2 and < 5 cm) needed to prevent local tumor progression: 3D quantitative assessment using CT image fusion. AJR Am J Roentgenol. 2010; 195:758–765.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download