Abstract
Objective
Materials and Methods
Results
Figures and Tables
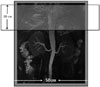 | Fig. 1Schematic picture of 2 orthogonal inversion bands localized geographically for displaying renal artery.Vertical IR band was firstly played and transversal IR band was performed closely after. IR = inversion recovery
|
 | Fig. 2Diagrams of relative signal change and respiratory triggered fat-sat 3D FIESTA sequence with SLEEK preparation.SLEEK = spatial labeling with multiple inversion pulses, FIESTA = fast imaging employing steady-state acquisition, TI = inversion time, 3D = three-dimensional
|
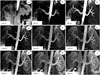 | Fig. 3Example of 26-year-old volunteer, who underwent SLEEK with various BSP TIs with 300 ms interval from 200 ms to 2600 ms.Renal artery was clearly visualized at BSP TI of 800 ms, 1100 ms, and 1400 ms (C-E). As BSP TI increased, inferior vena cava and renal vein were more distinctly visualized and renal artery was more obscured (F-I). As BSP TI decreased, abdominal aorta and renal artery was unclearly presented (A, B). BSP TI = blood suppression inversion time, SLEEK = spatial labeling with multiple inversion pulses
|
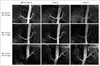 | Fig. 4Assessment of renal artery image quality in SLEEK MR angiography with various BSP TIs (800 ms, 1100 ms, 1400 ms) according to different median BRs and HRs in 3 hypertensive patients.For first subject (BR = 18/min; HR = 78 bpm), SLEEK MR angiography shows best image quality at BSP TI of 800 ms. For second patient (BR = 15/min; HR = 73 bpm), SLEEK MR angiography presented best image quality at BSP TI of 1100 ms. For third patient (BR = 14/min; HR = 66 bpm), SLEEK MR angiography displayed best image quality at BSP TI of 1400 ms. Those images indicated that optimal BSP TI had negative correlation with BR and HR and could be helpful to boost renal arterial visibility, especially for renal artery branch in renal parenchyma. bpm = beats per minute, BR = breathing rate, BSP TI = blood suppression inversion time, HR = heart rate, SLEEK = spatial labeling with multiple inversion pulses
|
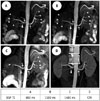 | Fig. 5Case 40-year-old hypertensive woman suspected of renal artery stenosis with optimal BSP TI of 800 ms at BR of 17-18/min and HR of 77-80 bpm.SLEEK MR angiography shows best image quality at BSP TI = 800 ms (A) than with BSP TI = 1100 ms (B) and BSP TI = 1400 ms (C), with distinct presentation of renal arterial branches (short arrows). For delineating bilateral renal artery stenosis (long arrows) and right accessory renal artery (arrowhead), they were not different and grade 2 stenosis in right renal artery was identified in (A), (B), and (C) as visualized with CTA (D). However, grade 1 stenosis in left renal artery confirmed with CTA (D) was overestimated by SLEEK MR angiography in (A), (B), and (C). BR = breathing rate, BSP TI = blood suppression inversion time, CTA = computed tomography angiography, HR = heart rate, SLEEK = spatial labeling with multiple inversion pulses
|
 | Fig. 6Case of 28-year-old woman with hypertension for > 10 years and renal artery stenosis due to fibromuscular dysplasia with optimal BSP TI = 1100 ms at BR of 14-15/min and HR of 74-76 bpm.SLEEK MR angiography shows best image quality at BSP TI = 1100 ms (B) than with BSP TI = 800 ms (A) and BSP TI = 1400 ms (C) with distinct presentation of renal arterial branches (short arrows) and "string-of-beads" vessel appearance (long arrow). D. Enlarged SLEEK image more clearly delineates appearance of "string-of-beads" in renal arterial branches (long arrows), as demonstrated in DSA (E) (long arrow). bpm = beats per minute, BR = breathing rate, BSP TI = blood suppression inversion time, DSA = digital subtraction angiography, HR = heart rate, SLEEK = spatial labeling with multiple inversion pulses
|
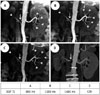 | Fig. 7Case of 54-year-old woman with hypertension for 8 years suspected of RAS with optimal BSP TI = 1100 ms at BR of 13-14/min and HR of 68-70 bmp.SLEEK MR angiography shows best image quality at BSP TI = 1400 ms (C) than at BSP TI = 800 ms (A) and BSP TI = 1100 ms (B) with distinct visualization of renal arterial branches (short arrows); position and degree of renal artery stenosis (long arrow) was no different among (A), (B), and (C), which was confirmed as grade 3 stenosis with CTA (D). bpm = beats per minute, BR = breathing rate, BSP TI = blood suppression inversion time, CTA = computed tomography angiography, HR = heart rate, RAS = renal artery stenosis, SLEEK = spatial labeling with multiple inversion pulses
|
Table 1
Image Quality of Renal Arteries by SLEEK NCE-MRA with Different BR and HR for Each Volunteer

"3" indicated best image (most SNR, most delineation of main renal artery and segmental branches in renal parenchyma, least interference from venous system); "2" represented better images (more SNR, more delineation of main renal artery and segmental branches in renal parenchyma, less interference from venous system); "1" implied good images (good SNR, delineation of main renal artery, some artifact may be present from venous system); "0" hinted poor images (low inhomogeneous signal intensity, incomplete delineation of main renal artery border, more artifact from venous system). bpm = beats per minute, BR = breathing rate, BSP TI = blood suppression inversion time, HR = heart rate, NCE-MRA = non-contrast enhanced magnetic resonance angiography, SLEEK = spatial labeling with multiple inversion pulses, SNR = signal-to-noise ratio
Table 2
Image Quality of SLEEK for All Hypertension Patients by Two Readers

| Image Quality Scores | ||||
|---|---|---|---|---|
| 3 | 2 | 1 | 0 | |
| Reader#1 | 58 (62.4%) | 30 (32.2%) | 2 (2.2%) | 3 (3.2%) |
| Reader#2 | 56 (60.2%) | 32 (34.4%) | 3 (3.2%) | 2 (2.2%) |
Table 3
Assessment of Renal Arteries with SLEEK by Two Readers in Comparison with Results of CTA and DSA

For those patients with FMD and bilateral renal artery disease, maximum stenotic degree was determined and recorded as stage. CTA = computed tomography angiography, DSA = digital subtraction angiography, FMD = fibromuscular dysplasia, RAS = renal artery stenosis, SLEEK = spatial labeling with multiple inversion pulses
Table 4
95% Confidence Intervals of BRs and HRs for Choosing Optimal BSP TI with Two Readers Respectively*





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download