Abstract
Objective
To evaluate the effectiveness of ultrasound and microbubble-liposome complex (MLC)-mediated delivery of siRNA and doxorubicin into prostate cancer cells and its therapeutic capabilities both in vitro and in vivo.
Materials and Methods
Microbubble-liposome complexes conjugated with anti-human epidermal growth factor receptor type 2 (Her2) antibodies were developed to target human prostate cancer cell lines PC-3 and LNCaP. Intracellular delivery of MLC was observed by confocal microscopy. We loaded MLC with survivin-targeted small interfering RNA (siRNA) and doxorubicin, and delivered it into prostate cancer cells. The release of these agents was facilitated by ultrasound application. Cell viability was analyzed by MTT assay after the delivery of siRNA and doxorubicin. Survivin-targeted siRNA loaded MLC was delivered into the xenograft mouse tumor model. Western blotting was performed to quantify the expression of survivin in vivo.
Results
Confocal microscopy demonstrated substantial intracellular uptake of MLCs in LNCaP, which expresses higher levels of Her2 than PC-3. The viability of LNCaP cells was significantly reduced after the delivery of MLCs loaded with siRNA and doxorubicin (85.0 ± 2.9%), which was further potentiated by application of ultrasound (55.0 ± 3.5%, p = 0.009). Survivin expression was suppressed in vivo in LNCaP tumor xenograft model following the ultrasound and MLC-guided delivery of siRNA (77.4 ± 4.90% to 36.7 ± 1.34%, p = 0.027).
Conclusion
Microbubble-liposome complex can effectively target prostate cancer cells, enabling intracellular delivery of the treatment agents with the use of ultrasound. Ultrasound and MLC-mediated delivery of survivin-targeted siRNA and doxorubicin can induce prostate cell apoptosis and block survivin expression in vitro and in vivo.
Prostate cancer is the most common non-skin cancer and the second leading cause of cancer mortality in the male population (1). Recently, new targeted gene therapy using RNA interference (RNAi) is being investigated for the treatment of advanced prostate cancer. The RNAi relies on post-transcriptional gene silencing using double-strand RNA processed into 21–25 nucleotide length, known as small interfering RNA (siRNA) (2). When siRNA is incorporated into RNA-induced silencing complexes in the cytoplasm, it promotes targeted gene silencing by sequence-specific degradation of messenger RNA (23). The siRNA is a promising therapeutic approach for cancer treatment, since it can prevent the production of specific proteins essential for the proliferation of tumor cells.
Many approaches have been studied to enhance the delivery of treatment agents such as siRNA into tumor cells (456). Our study group developed microbubble-liposome complex (MLC) for the guided delivery of agents to the prostate cancer cells (7). Microbubbles, gas bubbles 1–8 µm in diameter (8910), can act as cavitation nuclei that carry drugs or genes to target cells, enabling site-targeted treatment (8910). Further application of ultrasound can facilitate microbubble-mediated delivery, by the collapse of microbubbles, perforations in cell membranes, and increased permeability of regional capillaries, which allows large molecules to stream into the cells (9). This phenomenon, called sonoporation, can expedite the intracellular ingress of drugs or genes in ultrasound microbubble-mediated delivery (911). Additionally, we linked liposomes with microbubbles to carry not only siRNA but also chemotherapeutic drug–doxorubicin. In a novel approach, we conjugated anti-human epidermal growth factor receptor type 2 (Her2) antibodies with MLC to target prostate cancer cells that are known to express Her2 (12).
The purpose of our study was to evaluate the effectiveness of ultrasound and MLC-mediated delivery of siRNA and doxorubicin into prostate cancer cells and the therapeutic capability of this approach both in vitro and in vivo.
Human prostate cancer cell lines PC-3 and LNCaP were obtained from American Type Culture Collection (Manassas, VA, USA). Cells were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum at 37℃ in a humidified 5% CO2 incubator. Cells were harvested from subcultures, supplemented with 0.01% trypsin EDTA (Sigma-Aldrich, St. Louis, MO, USA), and re-suspended in fresh medium for experiments.
Survivin-targeted siRNA (Human BIRC5 [Gene ID 332], target sequence: CAAAGGAAACCAACAAUAA, GCAAAGGAAACCAACAAUA, CACCGCAUCUCUACAUUCA, CCACUGAGAACGAGCCAGA) was obtained from the siGENOME SMARTpool (Dharmacon Products, Thermo Fisher Scientific, Waltham, MA, USA), and adjusted according to the manufacturer's instruction. Doxorubicin (molecular weight, 579.98 g/mol) was purchased from Sigma-Aldrich, St. Louis, MO, USA.
Microbubble-liposome complexes were prepared as previously described (7). Lipid stocks (Avanti Polar Lipids, Albaster, AL, USA) comprising 15.4 mg of 1,2-dipalmitory-sn-glycero-3-phosphatidylcholine, 3.5 mg of cholesterol, 1 mg of dicetyl phosphate, 1.2 mg of 1,2-dipalmitory-sn-glycero-3-phosphoethanolamine, and 5 mg of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-(PDP[polyethylene glycol]-2000) were dissolved in 5 mL of 99.9% chloroform (Sigma-Aldrich). The mixture was lyophilized for 24 hours to remove chloroform, and mixed with 2 mL of solvent comprising glycerin, propylene glycol, and H2O (1:2:7). Cores of synthesized microbubbles were filled with sulfur hexafluoride gas (SF6). Liposomes were formed by freezing and thawing the mixture 5 times using liquid nitrogen, followed by agitation in a sonicator at 60℃ for 5 minutes with 2 mL of H2O. The resultant multilamellar vesicles were extruded using polycarbonate filters (filter size, 200 nm) to obtain liposomes smaller than 200 nm. Microbubbles and liposomes were shaken together at 25℃ for 2 hours to form MLC. Sulfosuccinimidyl-4-(N-maleimidomethyl) cyclohexane-1-carboxylate (5 mg; Sigma-Aldrich) was added to MLCs, followed by conjugation with anti-Her2 antibodies (Herceptin®; Roche, Berlin, Germany) by shaking at 4℃ for 24 hours to allow MLCs to target Her2-expressing prostate cancer cells (12).
Microbubble-liposome complexes were labeled using fluorescein isothiocynate (Sigma-Aldrich) for microbubbles to emit green fluorescence, and Texas red (Sigma-Aldrich) for liposomes to emit red fluorescence. PC-3 and LNCaP cells were seeded in 1-well chamber slides (1 × 105 cells/well). The cells were subsequently mixed with MLCs and incubated for 3 hours at 37℃.
After washes with cold phosphate buffered saline (PBS), the cells were exposed to ultrasound waves using an ultrasound scanner (Philips Medical Systems, Bothell, WA, USA) with a linear probe. The cells were seeded in a line in the chamber slide, and submerged in buffered saline. Ultrasound exposure ("US-flashing") was performed by applying ultrasound waves ranging in frequency from 5 to 12 MHz over the cell chambers, with an interval of 1 second for 2 minutes and a mechanical index of 0.61.
Transfected cells were incubated for 3 hours at 37℃, and the intracellular MLCs were visually localized using a confocal laser scanning microscopy at × 400 magnifications (Leica Microsystems, Wetzler, Germany).
Microbubble-liposome complexes were loaded with survivin-targeted siRNA and/or doxorubicin in 4 formulations: 1) unloaded MLCs, 2) MLCs with doxorubicin (Dox-MLCs), 3) MLCs with siRNA (siRNA-MLCs), and 4) MLCs with siRNA and doxorubicin (Dox-siRNA-MLCs) by adding 5 nmoL of siRNA and/or 1 mg of doxorubicin to MLCs and dissolving the combination in 2 mL of H2O. After freezing, thawing, and agitation, final complexes were filtered using 200-nm polycarbonate filters.
Before doxorubicin loading, ultraviolet (UV) absorbance standard curve was obtained using a UV-visible (UV-vis) spectrophotometer (Scinco, Seoul, Korea). After loading, Dox-MLCs were separated from the free drug by centrifugation at 13000 rpm, with the resultant supernatant analyzed by UV-vis spectrophotometry. UV detection was maximal at 480 nm. The loading efficiency was calculated using the following equation: loading efficiency ([initial drug concentration - drug concentration in the supernatant] / initial drug concentration) × 100%.
PC-3 and LNCaP cells were seeded in a 96-well chamber slide at 2.0 × 103 cells/well. The cells were treated with 4 different methods: 1) no treatment (group 1), 2) Dox-siRNA-MLCs loading (group 2), 3) US-flashing without MLCs (group 3), and 4) Dox-siRNA-MLCs loading with US-flashing (group 4). For groups 2 and 4, Dox-siRNA-MLCs were added to each well, and the cells were incubated for 3 hours at 37℃. The cells in group 3 and 4 underwent US-flashing using the parameters described earlier.
Clonogenic MTT assay was performed immediately following treatment (day 0) and after 3 days of incubation (day 3). MTT reagent (50 µL; Sigma-Aldrich) was added to the treated cells, followed by 150 µL of DMSO (Sigma-Aldrich) and incubated for 3 hours at 37℃. Cell plates were shaken for 1 hour to dissolve MTT crystals. Optical density at 450 nm was read in a spectrophotometer (Scinco, Seoul, Korea), and cell viability was calculated relative to the control group.
The effect of each therapeutic agent (survivin-siRNA and doxorubicin) with or without ultrasound exposure was assessed by MTT assay. PC-3 and LNCaP cells (2.0 × 103 cells/well) were seeded in 96-well chambers. The cells were treated with 1) no additives, 2) unloaded MLCs, 3) Dox-MLCs, 4) siRNA-MLCs, or 5) Dox-siRNA-MLCs. A separate group of cells underwent same treatment, with the addition of US-flashing performed as previously described. After 3 days of incubation at 37℃, viability of treated cells was evaluated using the MTT assay.
All animal protocols were approved by the Institutional Animal Care and Use Committee. PC-3 and LNCaP cells (1.5 × 106 cells in 0.2 mL of PBS) were subcutaneously injected in both flanks of 6 athymic nude male mice (n = 3 for each cell type) from an animal facility (Orient, Seoul, Korea) to produce xenografts of prostate tumor model.
After 4 to 6 weeks of tumor growth, mice were euthanized with isoflurane. One mouse with a PC-3 tumor and one with an LNCaP tumor were used as controls without any treatment. Two mice in each group were injected 0.2 mL of Dox-siRNA-MLC dissolved in PBS via tail vein. All MLCs were fluorescence-labeled with Texas red. Following the injection, US-flashing was performed for 5 minutes with an interval of 3 seconds with the mechanical index of 0.47 on the tumors in the right flank (Fig. 1). US-flashing was not applied to the left flank tumor, to allow the two tumors to be compared within the same animal.
After 24 hours, mice were sacrificed and tissue sections were obtained from tumors on each side of the animal. Tumor uptake of Dox-siRNA-MLC was assessed by confocal laser scanning microscopy at × 400 magnifications and survivin expression was quantified by Western blot analysis.
Tissue samples were homogenized in 600 µL of PROPREPTM Protein Extraction solution (Intron Biotechnology, Seoul, Korea). After centrifugation at 13000 rpm for 10 minutes at 4℃, 20 µg of supernatant was added to a 5 × SDS gel-loading buffer. The sample solution was boiled at 100℃ for 5 minutes, loaded onto the SDS gel, and electrophoresis was performed for 20 minutes at 80 V and 60 minutes at 130 V. Proteins were transferred to a membrane in transfer buffer at 80 V for 1.5 hours. The membrane was blocked with 5% skim milk in Tris-buffered saline with Tween (TBS-T) solution for 30 minutes at room temperature, and incubated with a diluted solution of primary antibody (anti-survivin, 1:2000 dilution; β-actin, 1:10000 dilution) overnight at 4℃. Following washing in TBS-T, the membrane was incubated with secondary antibody solution (anti-rabbit, 1:2000 dilution) for 1 hour at room temperature. Proteins of interest were detected using WEST-ZOL® Western Blot Detection System (Intron Biotechnology, Seoul, Korea). Survivin expression was normalized to β-actin levels, and the ratio of survivin expression relative to β-actin was calculated.
Data were expressed as means ± standard deviations. Differences between multiple experimental groups were compared using Kruskal-Wallis tests followed by post-hoc tests with Bonferroni correction. Comparisons between two experimental groups were performed with Mann-Whitney or Wilcoxon signed rank tests. Statistical analyses were performed using statistical software (SPSS, version 18.0; SPSS Inc., Chicago, IL, USA). p values < 0.05 were considered statistically significant.
No substantial fluorescence was observed before and after US-flashing in PC-3 cells that have relatively low Her2 expression (Fig. 2A). Conversely, LNCaP cells, which are known to express higher levels of Her2 than PC-3 cells, showed substantial green and red fluorescence, indicating the presence of labeled microbubbles and liposomes after incubation with the mix of MLCs, both before and after US-flashing (Fig. 2B).
The efficiency of doxorubicin loading was determined as 61.9%, with the total concentration of loaded doxorubicin of 213.6 µM. The concentration of loaded doxorubicin per treated cell well was 21.4 µM.
Figure 3A summarized the cell survival data acquired following different treatments.
In PC-3 cells (Fig. 3B), cell survival rate was determined as > 90% in all treatment groups on Day 0. While cell survival rate was reduced by 4% in group 4 on Day 3, no statistically significant difference from Day 0 was observed (Wilcoxon signed rank test, p = 0.25). The other 3 groups also did not show any significant alterations in cell viability on Day 3 (Wilcoxon signed rank test, group 1, p = 0.73, group 2, p = 0.46, group 3, p = 0.05).
In LNCaP cells (Fig. 3C), cell viability on Day 0 was > 90% in all treatment groups. After 3 days of incubation, group 4 (cells treated with Dox-siRNA-MLCs and US-flashing) showed a significant reduction in cell viability (94.2 ± 2.8%, vs. 41.8 ± 3.2%, on Days 0 and 3, respectively; Wilcoxon signed rank test, p = 0.009). Cell viability was significantly different between the 4 treatment groups on day 3 (Kruskal-Wallis test, p = 0.006). In subgroup analysis, cell viability of group 4 was significantly lower than in group 1 (Mann-Whitney U test, p = 0.005).
Figure 4A summarized the cell viability data in subgroups
of PC-3 and LNCaP cells.
Cell survival rate in PC-3 cells was > 90% in all subgroups without US-flashing (Fig. 4B), which was not significantly altered in cells treated with additional US-flashing (all subgroups, Mann-Whitney U test, p > 0.05).
Viabilities in LNCaP cells treated with Dox-MLCs, siRNA-MLCs, or Dox-siRNA-MLCs were significantly lower in groups with US-flashing (Dox-MLCs, 88.0 ± 3.4% vs. 63.0 ± 1.8%, p = 0.009; siRNA-MLCs, 87.0 ± 4.1% vs. 73.0 ± 3.8%, p = 0.009; Dox-siRNA-MLCs, 85.0 ± 2.9% vs. 55.0 ± 3.5%, p = 0.009, Mann-Whitney U test). The rate of reduction of cell viability was highest in cells treated with Dox-siRNA-MLCs and US-flashing, followed by the cells treated with Dox-MLCs and US-flashing, and siRNA-MLCs and US-flashing (Fig. 4C). The viability in cells treated with Dox-siRNA-MLCs and US-flashing was significantly lower than in cells treated with Dox-MLCs and US-flashing (Mann-Whitney U test, p = 0.008).
In PC-3 xenograft tumor model, no substantial uptake of Dox-siRNA-MLCs in the tumor was observed by confocal microscopy (Fig. 5A). In contrast, substantial red fluorescence reflecting tumor uptake of Dox-siRNA-MLCs was observed in LNCaP tumor, which became more pronounced after US-flashing (Fig. 5B).
In Western blot analysis, the levels of survivin expression were lower in LNCaP tumors treated with Dox-siRNA-MLC compared to the control, and lowest in LNCaP tumors treated with both Dox-siRNA-MLC and US-flashing. Survivin expression ratios were 77.4 ± 4.90% in control tissues, 52.7 ± 2.83% in LNCaP tumors with Dox-siRNA-MLCs, and 36.7 ± 1.34% in LNCaP tumors with Dox-siRNA-MLCs and US-flashing (Kruskal-Wallis test, p = 0.027; all subgroups, Mann-Whitney U test, p = 0.10) (Fig. 5C, D). Survivin expression in the treated PC-3 tumors was decreased to a lesser degree (control, 63.1 ± 4.36%; PC-3 tumor with Dox-siRNA-MLCs, 56.8 ± 4.35%; PC-3 tumor with Dox-siRNA-MLCs and US-flashing, 56.6 ± 3.08%; Kruskal-Wallis test, p = 0.113; subgroup analysis, Mann-Whitney U test, p = 0.20, 1.0, 0.10, respectively).
Figure 6 depicted schematically ultrasound and MLC-mediated delivery of survivin-targeted siRNA and doxorubicin into prostate cancer cells. We demonstrated that MLCs could effectively target prostate cancer cells expressing Her2 by the conjugation of anti-Her2 antibodies. We successfully loaded MLCs with survivin-targeted siRNA and doxorubicin, and delivered into prostate cancer cells in vitro and prostate tumor model in vivo. Additional ultrasound exposure escalated the therapeutic effects of the loaded substances.
Most prostate cancers tend to recur as aggressive androgen-refractory prostate cancers (1314). While chemotherapeutic drugs have improved the survival rate (15), no reliable therapeutic method for androgen-refractory prostate cancer has yet been established. In this regard, siRNA in prostate cancer therapy has been actively investigated over the years. Survivin, an anti-apoptotic protein (1617) strongly expressed in prostate cancer cells (18), has been identified as an attractive therapeutic target. It is associated with cellular proliferation (19), resistance to androgen deprivation therapy, and metastasis of prostate cancer (1920). The siRNA-mediated down-regulation of survivin can reduce prostate cancer cell proliferation, stimulate apoptosis (1617), and increase the chemosensitivity (2122).
However, intracellular delivery of siRNA is hindered by its fast degradation in the physiologic environment and its inability to cross the cell membrane (45). Additive "carriers" must therefore be attached to siRNA to achieve effective delivery. These carriers need to protect siRNA from degradation, transport it to the target cells, and release it into the cytoplasm without toxicity (46). MLCs contain microbubbles that function effectively as carriers, and can target specific tissues by incorporating target-specific ligands (8). Plus, ultrasound can facilitate the delivery of molecules by sonoporation (89). Additional disruption of microbubbles by ultrasound energy can increase cell permeability (23), while tissue-selective targeting also can be achieved by focusing ultrasonic field (10). Thus, ultrasound MLC-mediated delivery can be a potent tool for target-specific therapy in prostate cancer.
One novel finding of our study was from the attachment of anti-Her2 antibodies to MLCs. The cellular level approach of the cancer treatment necessitates proper targeting of cancer cells. Thus, specific targeting method suitable for prostate cancer cells is required. Since it is well known that prostate cancer cells express Her2 (12), we aimed to facilitate the ability of MLCs to target prostate cancer cells by conjugating anti-Her2 antibodies. As a result, intracellular uptake of MLCs was significantly higher in LNCaP cells that express higher levels of Her2 than PC-3 cells. This proved that MLCs conjugated with anti-Her2 antibodies could effectively target prostate cancer cells.
The cytotoxic effect was accentuated with the delivery of MLCs containing both doxorubicin and survivin-targeted siRNA, as compared to the MLCs containing doxorubicin alone. Knock-down of survivin was previously shown to increase the chemosensitivity of prostate cancer (2122). Therefore, co-delivery of chemotherapeutic agent with siRNA can elicit a synergistic effect in prostate cancer treatment. Since MLCs can deliver both siRNA and doxorubicin, it can be an effective tool for targeted cancer therapy.
Targeting of MLCs and their capacity to deliver siRNA and doxorubicin was verified in a xenograft prostate tumor model as well. Suppression of survivin expression was observed in the tumor tissue, especially with the application of ultrasound waves. This result suggested that the delivery of siRNA and doxorubicin could be improved by ultrasound-induced microbubble burst, both in vitro and in vivo.
There are a few limitations in our study. First, fluorescent imaging was performed 3 hours following the start of incubation, which could result in an underestimation of the total MLC uptake, since the delayed uptake would be excluded from our analysis. Further studies need to analyze the delayed uptake following sonoporation to support our findings. Second, a small number of mice were included in vivo analysis, precluding any statistical analysis. While our observations demonstrate the feasibility of in vivo application of ultrasound MLC-mediated delivery of therapeutic agents, further studies are needed to validate our results.
In conclusion, MLCs loaded with specific ligand are an effective tool for intracellular delivery of siRNA and doxorubicin into prostate cancer cells in vitro and into a prostate tumor model in vivo. With the application of ultrasound, MLCs containing survivin-targeted siRNA and doxorubicin elicited a cytotoxic effect and inhibited the expression of survivin. Therefore, ultrasound MLC-mediated delivery of siRNA and chemotherapeutic agents opens up the possibility of clinically applicable image-guided therapy and provides novel prospects for therapeutic applications in the near future.
Figures and Tables
 | Fig. 1Ultrasound images of xenograft prostate tumors.Sonography shows solid LNCaP prostate tumor mass in right flank of mouse on contrast-specific mode with pure harmonic detection (A) and on grey scale mode (B).
|
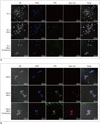 | Fig. 2Confocal laser scanning microscopy images of PC-3 cells and LNCaP cells.
A. Confocal microscopy images reveal no visible fluorescence in cells (× 400 magnification), suggesting poor uptake of MLCs into PC-3 cells. B. Green fluorescence in cells labeled by FITC and red fluorescence in cells labeled by Texas red are observed under microscopy (× 400) before and after ultrasound exposure. Observed fluorescence patterns suggest that microbubble-liposome complexes (MLCs) conjugated with anti-Her2 antibodies efficiently target LNCaP cells. Her2 = human epidermal growth factor receptor type 2
|
 | Fig. 3Cell viability after treatment of PC-3 cells and LNCaP cells with microbubble-liposome complexes (MLCs).
A. Bar graph depicting viability of PC-3 and LNCaP cells, demonstrating that LNCaP cells treated with Dox-siRNA-MLCs followed by ultrasound exposure show significant reduction in cell viability. B. Cell viability of PC-3 cells, evaluated by MTT assay on days 0 and 3, was not significant different between non-treated and treated groups. Dox-siRNA-MLCs = MLCs with siRNA and doxorubicin C. Cell viability of LNCaP cells, evaluated by MTT assay on days 0 and 3, was significantly reduced on day 3 in group treated with Dox-siRNA-MLCs followed by ultrasound exposure. Dox-siRNA-MLCs = MLCs with siRNA and doxorubicin
|
 | Fig. 4Effect of therapeutic agents and ultrasound guidance on viability of PC-3 and LNCaP cells.
A. Bar graph depicting viability of PC-3 and LNCaP cells, demonstrating that viability of LNCaP cells was decreased following ultrasound exposure when treated with Dox-MLCs (from 88.0 ± 3.4% to 63.0 ± 1.8%), siRNA-MLCs (from 87.0 ± 4.1% to 73.0 ± 3.8%), and Dox-siRNA-MLCs (from 85.0 ± 2.9% to 55.0 ± 3.5%). All decreases were statistically significant (p < 0.01). Dox-MLCs = MLCs with doxorubicin, Dox-siRNA-MLCs = MLCs with siRNA and doxorubicin, MLCs = microbubble-liposome complexes, siRNA-MLCs = MLCs with siRNA B, C. MTT assays performed with PC-3 and LNCaP cells show no significant difference in viability of PC-3 cells between treatment subgroups. Conversely, cell viabilities were decreased after ultrasound exposure in subgroups of LNCaP cells. Dox-MLCs = MLCs with doxorubicin, Dox-siRNA-MLCs = MLCs with siRNA and doxorubicin, MLCs = microbubble-liposome complexes, siRNA-MLCs = MLCs with siRNA
|
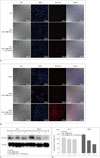 | Fig. 5Effect of treatment with microbubble-liposome complex (MLC) on PC-3 and LNCaP xenograft tumor models.
A. No fluorescence signal was observed in PC-3 tumor on confocal microscopy (× 400 magnification). Dox-siRNA-MLCs = MLCs with siRNA and doxorubicin B. Bright red fluorescence signal was observed in LNCaP tumor in confocal images (× 400), suggesting intra-tumor uptake of MLCs. It should be noted that amount of intra-tumor uptake of fluorescent MLCs after ultrasound exposure is increased after ultrasound exposure. C. Western blot analysis demonstrated reduced survivin expression in LNCaP cells treated with siRNA-loaded MLCs. Levels of expression of survivin are further decreased following ultrasound exposure. Protein expression was normalized to expression of β-actin. D. Bar graphs show mean survivin density in each treatment group. Mean survivin density is lower in treated LNCaP cells compared to control cells (group 1, 77.4 ± 4.90%; group 2, 52.7 ± 2.83%; group 3, 36.7 ± 1.34%; p = 0.027). No substantial decrease in density of survivin was observed in PC-3 cells (group 1, 63.1 ± 4.36%; group 2, 56.8 ± 4.35%; group 3, 56.6 ± 3.08%; p = 0.113). Dox-siRNA-MLCs = MLCs with siRNA and doxorubicin
|
 | Fig. 6Schematic depiction of ultrasound MLC-mediated intracellular delivery of survivin-targeted siRNA and doxorubicin.
A. PC-3 tumors, devoid of Her2 receptor, did not take up therapeutic materials even following exposure to ultrasound waves. B. LNCaP tumors, which express Her2 receptor, take up siRNA and doxorubicin into cells under exposure to ultrasound waves. Her2 = human epidermal growth factor receptor type 2, MLC = microbubble-liposome complex, siRNA = small interfering RNA
|
Acknowledgments
The authors thank the Medical Research Collaborating Center at Seoul National University Bundang Hospital for performing the statistical analyses.
References
1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012; 62:10–29.
2. Kawasaki H, Taira K, Morris KV. siRNA induced transcriptional gene silencing in mammalian cells. Cell Cycle. 2005; 4:442–448.
3. Barik S. Silence of the transcripts: RNA interference in medicine. J Mol Med (Berl). 2005; 83:764–773.
4. Hasan W, Chu K, Gullapalli A, Dunn SS, Enlow EM, Luft JC, et al. Delivery of multiple siRNAs using lipid-coated PLGA nanoparticles for treatment of prostate cancer. Nano Lett. 2012; 12:287–292.
5. Becker AL, Orlotti NI, Folini M, Cavalieri F, Zelikin AN, Johnston AP, et al. Redox-active polymer microcapsules for the delivery of a survivin-specific siRNA in prostate cancer cells. ACS Nano. 2011; 5:1335–1344.
6. Xue HY, Wong HL. Tailoring nanostructured solid-lipid carriers for time-controlled intracellular siRNA kinetics to sustain RNAi-mediated chemosensitization. Biomaterials. 2011; 32:2662–2672.
7. Yoon YI, Kwon YS, Cho HS, Heo SH, Park KS, Park SG, et al. Ultrasound-mediated gene and drug delivery using a microbubble-liposome particle system. Theranostics. 2014; 4:1133–1144.
8. Wang X, Liang HD, Dong B, Lu QL, Blomley MJ. Gene transfer with microbubble ultrasound and plasmid DNA into skeletal muscle of mice: comparison between commercially available microbubble contrast agents. Radiology. 2005; 237:224–229.
9. Blomley MJ, Cooke JC, Unger EC, Monaghan MJ, Cosgrove DO. Microbubble contrast agents: a new era in ultrasound. BMJ. 2001; 322:1222–1225.
10. Hernot S, Klibanov AL. Microbubbles in ultrasound-triggered drug and gene delivery. Adv Drug Deliv Rev. 2008; 60:1153–1166.
11. Wu Y, Unger EC, McCreery TP, Sweitzer RH, Shen D, Wu G, et al. Binding and lysing of blood clots using MRX-408. Invest Radiol. 1998; 33:880–885.
12. Malmberg J, Tolmachev V, Orlova A. Imaging agents for in vivo molecular profiling of disseminated prostate cancer: cellular processing of [(111)In]-labeled CHX-A"DTPA-trastuzumab and anti-HER2 ABY-025 Affibody in prostate cancer cell lines. Exp Ther Med. 2011; 2:523–528.
13. Ling YX, Tao J, Fang SF, Hui Z, Fang QR. Downregulation of Id1 by small interfering RNA in prostate cancer PC3 cells in vivo and in vitro. Eur J Cancer Prev. 2011; 20:9–17.
14. Denmeade SR, Lin XS, Isaacs JT. Role of programmed (apoptotic) cell death during the progression and therapy for prostate cancer. Prostate. 1996; 28:251–265.
15. Petrioli R, Paolelli L, Francini E, Manganelli A, Salvestrini F, Francini G. Weekly docetaxel and epirubicin in treatment of advanced hormone-refractory prostate cancer. Urology. 2007; 69:142–146.
16. Paduano F, Villa R, Pennati M, Folini M, Binda M, Daidone MG, et al. Silencing of survivin gene by small interfering RNAs produces supra-additive growth suppression in combination with 17-allylamino-17-demethoxygeldanamycin in human prostate cancer cells. Mol Cancer Ther. 2006; 5:179–186.
17. Koike H, Morikawa Y, Sekine Y, Matsui H, Shibata Y, Suzuki K. Survivin is associated with cell proliferation and has a role in 1a,25-dihydroxyvitamin D3 induced cell growth inhibition in prostate cancer. J Urol. 2011; 185:1497–1503.
18. McEleny KR, Watson RW, Coffey RN, O'Neill AJ, Fitzpatrick JM. Inhibitors of apoptosis proteins in prostate cancer cell lines. Prostate. 2002; 51:133–140.
19. Zhang M, Latham DE, Delaney MA, Chakravarti A. Survivin mediates resistance to antiandrogen therapy in prostate cancer. Oncogene. 2005; 24:2474–2482.
20. Zhang M, Coen JJ, Suzuki Y, Siedow MR, Niemierko A, Khor LY, et al. Survivin is a potential mediator of prostate cancer metastasis. Int J Radiat Oncol Biol Phys. 2010; 78:1095–1103.
21. Rahman KM, Banerjee S, Ali S, Ahmad A, Wang Z, Kong D, et al. 3,3'-Diindolylmethane enhances taxotere-induced apoptosis in hormone-refractory prostate cancer cells through survivin down-regulation. Cancer Res. 2009; 69:4468–4475.
22. Shen J, Liu J, Long Y, Miao Y, Su M, Zhang Q, et al. Knockdown of survivin expression by siRNAs enhances chemosensitivity of prostate cancer cells and attenuates its tumorigenicity. Acta Biochim Biophys Sin (Shanghai). 2009; 41:223–230.
23. Skyba DM, Price RJ, Linka AZ, Skalak TC, Kaul S. Direct in vivo visualization of intravascular destruction of microbubbles by ultrasound and its local effects on tissue. Circulation. 1998; 98:290–293.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download