INTRODUCTION
MATERIALS AND METHODS
Patients
Image Acquisition and Analysis
CCT Studies
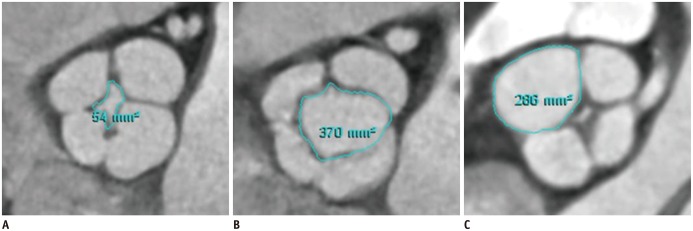 | Fig. 1Measurement of regurgitant orifice area (A) at mid-diastole, opening area (B) at early-systole, and individual valve cusp area (C) at mid-diastole in patient with quadricuspid aortic valve assessed by planimetry on cardiac computed tomography. |
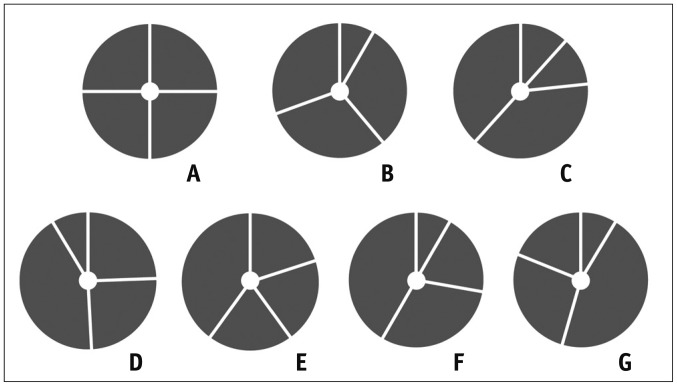 | Fig. 2Classification of quadricuspid aortic valve according to Hurwitz and Robert's classification.
A. Four equal cusps. B. Three equal cusps and one smaller cusp. C. Two equal larger cusps and two equal smaller cusps. D. One large, two intermediate, and one small cusp. E. Three equal cusps and one larger cusp. F. Two equal larger cusps and two unequal smaller cusps. G. Four unequal cusps. Adapted from Hurwitz and Roberts. Am J Cardiol 1973;31:623-626 (2).
|
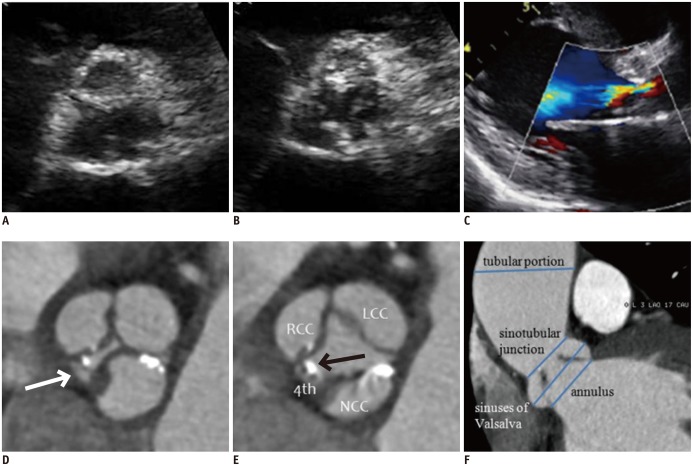 | Fig. 352-year-old man with Hurwitz and Robert's type F quadricuspid aortic valve combined with dilatation of ascending aorta.
A, B. Parasternal short-axis transthoracic echocardiographic images during diastole (A) and systole (B) show aortic valve. C. Parasternal long-axis color Doppler image during diastole shows moderate aortic regurgitation and moderate degree of aortic stenosis (mean pressure gradient = 38 mm Hg, not shown). D, E. Short-axis cardiac computed tomography (CCT) images of aortic valve during diastole (D) and systole (E) show thickened and calcified quadricuspid aortic valve with two equal larger cusps and two unequal smaller cusps. Areas of right cusp, left cusp, noncoronary cusp, and accessory cusp (arrows) are 2.44, 3.03, 2.92, and 0.31 cm2, respectively. Lack of coaptation of aortic valve (regurgitant orifice area = 0.1 cm2) was detected during diastole (D). F. Oblique coronal CCT image shows measurement of ascending aorta diameter at four different levels during early systole (annulus, 27 mm; sinuses of Valsalva, 36.7 mm; sinotubular junction, 26.7 mm; tubular portion, 51 mm). Patient underwent combined aortic valvuloplasty and ascending aorta wrapping. LCC = left coronary cusp, NCC = noncoronary cusp, RCC = right coronary cusp, 4th = accessory cusp
|
CMR Studies
TTE Studies
Statistical Analysis
RESULTS
Patient Population
Table 1
Classification, Functional Status, and Associated Condition in 11 Patients with Quadricuspid Aortic Valve
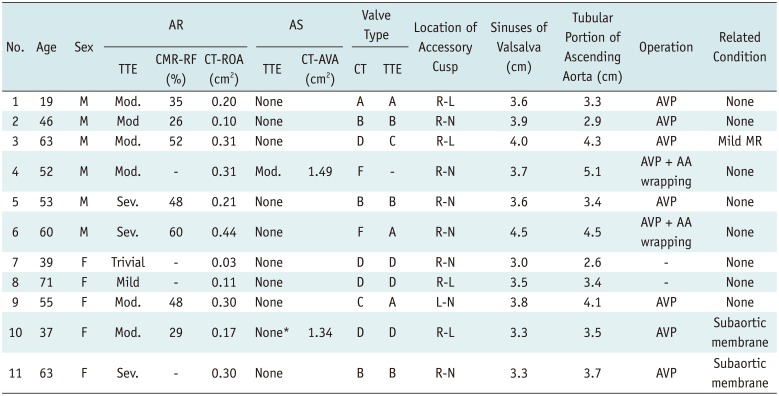
*TTE reveals severe subaortic stenosis with subaortic membrane without AS at valve level. AA = ascending aorta, AR = aortic regurgitation, AS = aortic stenosis, AVA = aortic valve area, AVP = aortic valvuloplasty, CMR = cardiovascular magnetic resonance, CT = computed tomography, L-N = left-noncoronary cusps, Mod. = moderate, MR = mitral regurgitation, R-L = right-left coronary cusps, R-N = right-noncoronary cusps, RF = regurgitant fraction, ROA = regurgitant orifice area, Sev. = severe, TTE = transthoracic echocardiography
TTE
CCT
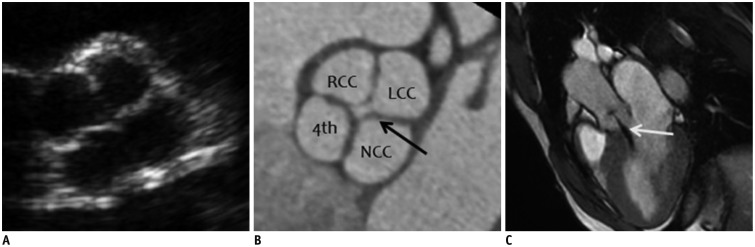 | Fig. 419-year-old man with Hurwitz and Robert's type A quadricuspid aortic valve (QAV) with four equal-sized cusps shown on transthoracic echocardiography (TTE), cardiac computed tomography (CCT), and cardiac magnetic resonance (CMR) imaging.
A. Parasternal short-axis TTE image of aortic valve during diastole shows QAV with four-leaf clover appearance. B. Short-axis CCT image of aortic valve during diastole demonstrates four equal aortic valve cusps. Areas of right cusp, left cusp, noncoronary cusp, and accessory cusp are 2.01, 2.13, 2.20, and 1.94 cm2, respectively. Lack of coaptation of aortic valve (arrow, regurgitant orifice area = 0.2 cm2) was detected during diastole, indicating aortic regurgitation. C. Oblique sagittal cine CMR image during diastole demonstrates central regurgitant jet (arrow) into left ventricular cavity. Quantitative analysis by phase-contrast CMR (not shown) yields regurgitant fraction of 35%, corresponding to moderate degree of aortic regurgitation (AR). LCC = left coronary cusp, NCC = noncoronary cusp, RCC = right coronary cusp, 4th = accessory cusp
|
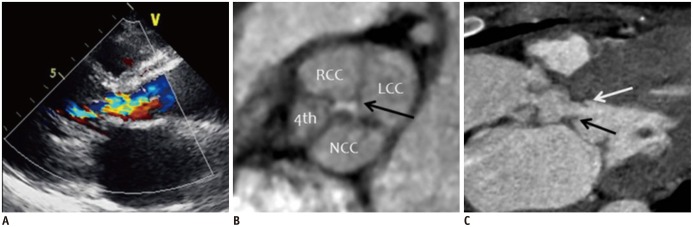 | Fig. 537-year-old woman with Hurwitz and Robert's type B quadricuspid aortic valve (QAV) combined with aortic regurgitation (AR) and subaortic stenosis due to subaortic membrane.
A. Parasternal long-axis color Doppler image during diastole shows moderate AR. B. Short-axis cardiac computed tomography (CCT) image of aortic valve during diastole demonstrates QAV with three equal-sized cusps and one smaller cusp. Areas of right cusp, left cusp, noncoronary cusp, and accessory cusp are 1.62, 2.13, 1.77, and 0.80 cm2, respectively. Lack of coaptation of aortic valve was detected (arrow, regurgitant orifice area = 0.17 cm2) during diastole. C. Oblique sagittal CCT image shows subaortic membrane (black and white arrows) with left ventricular outflow tract obstruction. Patient underwent aortic valvuloplasty and resection of subaortic membrane. LCC = left coronary cusp, NCC = noncoronary cusp, RCC = right coronary cusp, 4th = accessory cusp
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download