Abstract
11C-methionine (Met) positron emission tomography (PET) is one of the most commonly used PET tracers for evaluating brain tumors. However, few reports have described tips and pitfalls of 11C-Met PET for general practitioners. Physiological 11C-Met uptake, anatomical variations, vascular disorders, non-tumorous lesions such as inflammation or dysplasia, benign brain tumors and patient condition during 11C-Met PET examination can potentially affect the image interpretation and cause false positives and negatives. These pitfalls in the interpretation of 11C-Met PET images are important for not only nuclear medicine physicians but also general radiologists. Familiarity with the spectrum and pitfalls of 11C-Met images could help prevent unfavorable clinical results caused by misdiagnoses.
18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging for lesions in the brain often yields false-negative results, because the physiological uptake of 18F-FDG associated with high glucose metabolism in the brain can mask brain lesions. To overcome these difficulties, many PET tracers, including amino acid compounds that are hard to partition into the brain, have been developed. Among the various amino acid compounds for PET, 11C-methionine (Met) is one of the most commonly used PET tracers (1). Amino acids have special roles in the brain, which contributes to cerebral protein synthesis, intermediary metabolism, and interneuronal synaptic transmission. In addition, the essential amino acids, including Met, must be obtained from diets through intestinal absorption and released into the blood supply. Cerebrovascular endothelial cells express specific carriers that mediate the entry and efflux of amino acids across the luminal membranes of the blood-brain barrier (BBB) (2). The clinical use of 11C-Met PET for the imaging of brain tumors that has been reported is as follows: diagnostic accuracy, lesion extension or tumor grading assessment, biopsy or treatment planning, and prognosis or therapeutic response prediction (3). However, ideal methods for the assessment of 11C-Met PET images remain controversial, although many reports have described the use of visual assessment, tumor-to-normal (T/N) ratio, or the mean and/or maximum standardized uptake value (SUV) (3). Additionally, 11C-Met is not an ideal tracer because non-tumor lesions reportedly show increased uptake. It is important for nuclear medicine physicians/radiologists to be familiar with the imaging features and pitfalls of 11C-Met PET images. In this study, we describe the tips and pitfalls of 11C-Met PET imaging for intracranial lesions.
The pituitary gland, choroid plexus, and confluence of sinuses frequently appear in areas of physiological uptake. Lesions in these regions are occasionally overlooked or misinterpreted because these regions show relatively higher uptake than those in surrounding tissues. In particular, the pituitary uptake may depend on the hormonal status in female patients. Normal variants, such as hyperostosis or fibrous dysplasia, also show relatively high 11C-Met uptake, although the underlying mechanism remains unknown (Fig. 1). On the other hand, pineal cysts, choroid plexuses cysts, parahippocampal cysts, and Rathke's cleft cysts (Fig. 2) show no or low 11C-Met uptake. Magnetic resonance imaging (MRI) should precede 11C-Met PET to check the morphological anatomy of the central nervous system (CNS). The effect of aging on physiological uptake in the brain is limited, although SUV in the normal brains of children is slightly different from that in the normal brains of adults (4). Whether or not the patients should be in the fasting state before 11C-Met PET is also controversial. In patients with head and neck cancer, one study reported that food intake before 11C-Met PET can decrease the tumor SUV, although there is no robust evidence showing that the fasted state affects visual interpretation (5). In typical PET/computed tomography (CT), CT scans can be helpful for identifying lesions and detecting calcification. Additionally, PET/CT can be safely performed in patients with implanted electronic devices, patients for whom MRI is contraindicated, or patients who are allergic to contrast media.
Typical high-grade glioma is easily diagnosed by MRI. However, edema or a destroyed BBB in the boundary zone of tumors frequently modifies MRI findings. 11C-Met PET, as information complementary to that of MRI, is helpful for the delineation of tumors and estimation of histopathological components or cellular activity. The overall sensitivity of 11C-Met PET for gliomas, including both high- or low-grade gliomas, is estimated to be approximately 76–100% in various studies (3). In addition, the studies of only low-grade gliomas reported a sensitivity ranging from 65–85% (2). For assessment of the histopathological grade of cerebral glioma, 11C-Met PET does not show clear predictive value through semi-quantitative or visual analyses to date, because of a significant degree of overlap among tumors of several grades (3). However, 11C-Met uptake is usually higher in high-grade gliomas than in low-grade gliomas (Fig. 3). As an exception, gliomatosis cerebri, a high-grade glioma, is occasionally difficult to detect using 11C-Met PET because of infiltrative invasion into the white matter (Fig. 4). Additionally, CT images during PET/CT examination may provide clues for diagnosis, e.g., calcification of oligodendroglioma and oligoastrocytoma (Fig. 5). The diagnosis of rare low-grade glioma is problematic because of its slow growth and the difficulties in histological confirmation. Few reports describe occasional high 11C-Met uptake of pilocytic astrocytoma or pleomorphic xanthoastrocytoma, which may be pitfalls in the diagnosis of these tumors (6). 11C-Met PET for evaluating ependymomas is reported mainly for spinal lesions, and the ependymomas could be detected to a certain degree except for some small lesions (7).
The assessment of biopsy sites using 11C-Met PET is compared with that using MRI because brain tumors are histologically heterogeneous with different cancer grades and necrosis. Biopsy planning depending only on MRI can lead to inaccurate diagnosis. Various studies suggest an advantage of 11C-Met PET for identifying the best target for biopsy or for estimation of the tumor volume.
Several studies show that 11C-Met PET has advantages for distinguishing radiation necrosis from focal recurrence after radiotherapy (3). The mechanism of 11C-Met uptake in radiation necrosis is different from that in recurrence. Radiation injury leads to passive diffusion across the broken BBB. Theoretically, uptake by recurrent tumors should exceed the uptake after radiation injury; however, the possibility of overlap between these uptakes exists. Actually, different T/N cutoff ratios (1.27–1.9) are also used for this indication. Previous studies report sensitivities ranging from 75–100% and specificities from 60–100% (3).
Apparently, 11C-Met PET is not useful for the diagnosis of brain metastasis in baseline surveys. Fine nodular metastases are difficult to detect using 11C-Met PET because of the resolution limits and lesion sizes. Primary CNS lymphoma (PCNSL) usually presents with various types of 18F-FDG uptakes and a wide range of appearances from massive lesions to faint abnormal signals on MRI. 11C-MET PET reportedly shows a higher sensitivity similar to that of 18F-FDG PET, for the detection of primary lesions in patients with PCNSL (8). However, some cases of PCNSL are more clearly detected using 11C-Met PET and contrast-enhanced MRI than using 18F-FDG PET (Fig. 6). In our experience, malignancies under an immunosuppressive state, such as in acquired immunodeficiency syndrome (AIDS) or post-chemotherapy, occasionally present low 11C-Met uptake of the tumor (Fig. 7). In patients with CNS germinoma, the tumor-contouring ability of 11C-Met PET for some malignant tumors is higher than that of 18F-FDG PET. Importantly, the visual appearance of CNS germinomas differs depending on their location. Suprasellar and pineal lesions are clearly shown to have higher uptakes than those of surrounding structures. On the other hand, lesions at the basal ganglia show higher asymmetrical uptakes (Fig. 8) (9). The 11C-Met uptake of several rare low-grade brain tumors has a confusing appearance similar to the images of high-grade gliomas. Therefore, morphological imaging, such as MRI, is required. Biological factors that affect 11C-Met uptake in these benign tumors that can provide fundamental information for interpreting 11C-Met PET are not fully examined. Mixed neuronal and glial tumors, such as gangliogliomas and dysembryoplastic neuroepithelial tumors (DNTs), are common brain tumors accompanied by epileptogenic foci. Previous studies show that gangliogliomas (Fig. 9), including gangliocytoma of Lhermitte-Duclos disease, show higher 11C-Met uptake than DNTs (Fig. 10), although their uptakes slightly overlap (10). Meningiomas, which are the most common primary brain tumors, also show high 11C-Met uptake. 11C-Met PET can be used to evaluate recurrent or residual meningiomas post-resection. However, according to a previous study, 11C-Met uptake of meningiomas is correlated with tumor volume and location, e.g., skull-base tumors have higher uptakes than tumors at other locations but are not correlated with tumor aggressiveness, such as MIB-1 index, tumor-doubling time, or tumor grade (11). Central neurocytoma, which is a representative benign tumor, is described as having a markedly higher 11C-Met uptake (Fig. 11) (12). 11C-Met PET in patients with pituitary adenoma, particularly in hormone-secreting adenomas, is used to detect recurrent lesions. Craniopharyngioma also is also reported to show mild 11C-Met uptake. Although the evidence of 11C-Met uptake for benign tumors is limited, it should be obtained to enable efficient management.
11C-Met PET imaging for vascular lesions in the brain often yields false-negative results. Increased 11C-Met uptake in the perivascular mononuclear infiltrate and gliotic reaction in the collagen capsule surrounding hematomas (Fig. 12), BBB breakdown of infarction, and plugged venous flow of malformation (Fig. 13) are potential mechanisms for positive 11C-Met findings (1314). To prevent misdiagnosis and unnecessary surgical intervention, MRI or CT should precede any PET examinations for evaluating cranial lesions.
Several reports speculate that the mechanism of 11C-Met uptake in inflammation involves increased metabolism and active amino acid transport as a result of the increased density of inflammatory cells, as well as disruption of BBB. Tumefactive demyelination is a representative disease that shows various uptake patterns depending on the disease activity. In addition, 11C-Met uptake patterns may not necessarily correspond to the enhanced patterns of the lesions (Fig. 14). Encephalitis exhibits various uptake patterns and degrees even in the same pathogens. 11C-Met uptake in encephalitis is relatively dependent on the disease activity, although the mechanisms of increased 11C-Met uptake remain unknown. Sarcoidosis, brain abscess, progressive multifocal leukoencephalopathy, and tuberculoma are also reported as causes of false positives in 11C-Met PET (15). In patients with AIDS, 11C-Met uptake may help distinguish lymphoma from toxoplasmosis. However, several cases of toxoplasmosis show high uptake similar to that of tumors (Fig. 15) (15). Current evidence cannot support the usefulness of 11C-Met PET for inflammation diseases.
Focal cortical dysplasia (FCD) or cortical tubers are representative dysplasias having epileptogenic foci. In general, MRI and 18F-FDG PET usually precede 11C-Met PET to detect epileptogenic focus. 11C-Met uptake of FCD is similar to the uptake of other gray matter, a finding that can be helpful for distinguishing FCD from ganglioglioma prior to surgery (10). However, we occasionally encounter FCD lesions with slightly higher uptake than that of the surrounding cortex (Fig. 16) (16). Modification of amino acid transporters, such as by gliotic reaction or hyperperfusion, is a proposed mechanism for positive 11C-Met uptake in FCD, although a concrete evidence of the mechanism remains unclear. Hamartoma, which is a benign neoplastic heterotrophic lesion, shows uptake similar to that of white matter, a finding that may contribute to accurate diagnosis (Fig. 17).
Intracranial lesions show a wide spectrum of 11C-Met PET/CT imaging features. Interpretation of 11C-Met PET images requires a high degree of suspicion for the presence of brain tumors. Familiarity with the characteristics and pitfalls of 11C-Met PET/CT imaging will contribute to better interpretation of findings by radiologists.
Figures and Tables
Fig. 1
52-year-old woman with epilepsy.
A. Axial 11C-methionine (Met) positron emission tomography/computed tomography (CT) image showing high 11C-Met uptake in bilateral thickened frontal bone (arrows). B. Axial CT bone window showing hyperostosis (arrows). Hyperostosis occasionally shows high 11C-Met uptake.
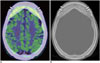
Fig. 2
75-year-old man with amygdala enlargement and Rathke's cleft cyst.
Sagittal 11C-methionine (Met) positron emission tomography/CT image showing no 11C-Met uptake in Rathke's cleft cyst, whereas physiological 11C-Met uptake is observed in pituitary gland in anterior part.
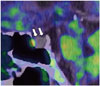
Fig. 3
76-year-old man with brain tumor.
A. Coronal 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT image showing lower uptake of convex lesion than of cortex (arrow). Lesion in white matter at right temporal lobes (arrowhead) shows markedly low uptake. B. Coronal 11C-methionine (Met) PET/CT image showing higher uptake at convex lesion (arrow), with tumor-to-normal (T/N) ratio of 7.2. However, lesion at right temporal lobes and opaque part showing lower 11C-Met uptake (arrowhead), with T/N ratio of 2.3. C. Axial contrast-enhanced MR T1-weighted imaging showing contrast-enhanced lesions in convex (arrow). Lesion at right temporal lobes (arrowhead) shows no contrast-enhancing. In biopsy specimens, high- and low-11C-Met-uptake areas corresponded to high- and low-grade astrocytomas.
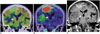
Fig. 4
48-year-old man with gliomatosis cerebri.
A. Axial fluid-attenuated inversion recovery image of magnetic resonance imaging showing high intensity with unclear boundaries in tegmentum of pons and bilateral internal portion of cerebellum (arrows). Lesion shows no contrast enhancement. B. Axial 11C-methionine (Met) positron emission tomography/CT showing unclear uptake of lesion, which is almost indistinguishable from background uptake in cerebellum (arrows), with tumor-to-normal ratio of 1.2. 11C-Met uptake appears vague or patchy in lesions.
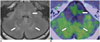
Fig. 5
26-year-old woman with anaplastic oligoastrocytoma and coarse calcifications.
Axial 11C-methionine positron emission tomography (PET)/CT image showing high uptake in lesion with coarse calcifications (arrows) at upper right frontal lobe, with tumor-to-normal ratio of 3.9. CT imaging during PET/CT is helpful for diagnosis of brain lesions.
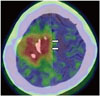
Fig. 6
84-year-old woman with primary central nervous system lymphoma.
A. Contrast-enhanced T1-weighted imaging (T1WI) of MRI showing contrast-enhanced lesions in left basal ganglia and bilateral cerebral peduncles (arrows). B. Axial 11C-methionine (Met) positron emission tomography (PET)/CT images clearly showing high uptakes in left basal ganglia and bilateral cerebral peduncles (arrows). Area of 11C-Met uptake is more widely spread out than that of contrast-enhanced T1WI. C. Lesion is poorly defined on coronal 18F-fluorodeoxyglucose PET/CT because of physiological uptake.
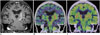
Fig. 7
56-year-old man with recurrent leukemia after chemotherapy.
A. Axial fluid-attenuated inversion recovery image of MRI showing high intensity lesion at right basal ganglia (arrow) without contrast enhancement. B. Axial 11C-methionine positron emission tomography/CT images showing low uptake corresponding to lesion (arrow), with tumor-to-normal ratio of 1.5. C. Postmortem brain specimen showing ring-like lesion, which was confirmed as infiltration of leukemia cells (arrow).
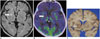
Fig. 8
15-year-old man with germinoma.
A. Contrast-enhanced T1-weighted image showing slightly enhanced lesions at left basal ganglia (arrows). B. Axial 11C-methionine positron emission tomography/CT image showing mild uptake in lesion (arrows).
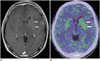
Fig. 9
2-year-old girl with ganglioglioma.
A. Axial 18F-fluorodeoxyglucose positron emission tomography (PET)/CT image showing lower uptake at cystic lesion surrounding solid portion with vague calcification at right temporal lobe (arrow). B. Axial 11C-methionine PET/CT image showing high uptake at solid component (arrow).
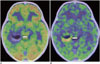
Fig. 10
5-year-old boy with dysembryoplastic neuroepithelial tumors.
A. T2-weighted image (T2WI) showing high-intensity mass with relatively distinct border at right temporal lobe (arrows). B. Axial 11C-methionine positron emission tomography/CT image showing low uptake corresponding to lesion with T2WI high-intensity area (arrows).
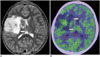
Fig. 11
22-year-old woman with central neurocytoma.
A. Axial CT image showing tumor with coarse calcifications in lateral ventricle (arrows). B. Axial 11C-methionine positron emission tomography/CT image showing high uptake at tumor (arrows), with tumor-to-normal ratio of 4.9.
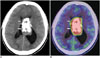
Fig. 12
26-year-old man with hematoma.
A. Axial T2-weighted image showing high-intensity mass at left temporal lobes (arrows). B. Axial 11C-methionine positron emission tomography/CT image showing low uptake at subcortical area surrounding slightly high uptake at cortex in left frontal lobe (arrows).
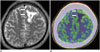
Fig. 13
25-year-old woman having venous malformation with plugged venous flow.
A. Axial 11C-methionine positron emission tomography/CT image showing high uptake at left basal ganglia (arrows), with tumor-to-normal ratio of 2.6. B. Axial contrast-enhanced CT image showing vascular anomalies in left basal ganglia (arrows). C. Volume-rendered CT angiographic image showing venous malformation at left basal ganglia (arrow).

Fig. 14
64-year-old man with tumefactive demyelination in post-treatment state.
A. Axial contrast-enhanced T1-weighted image showing lesion with non-enhancement of deep white matter in left posterior temporal lobe (arrow). B. Axial 11C-methionine positron emission tomography/CT image taken at almost same time as in (A) showing high uptake corresponding to lesion (arrow).
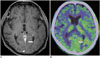
Fig. 15
28-year-old man with toxoplasmosis caused by acquired immunodeficiency syndrome.
A. Axial contrast-enhanced T1-weighted image showing clearly enhanced lesion with edema (arrow) in right parietal lobe. B. Coronal 11C-methionine positron emission tomography/CT image showing high uptake (arrow) corresponding to lesion, with tumor-to-normal ratio of 4.0.
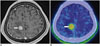
Fig. 16
25-year-old woman with focal cortical dysplasia.
A. Axial T2-weighted image showing slight thickening in cortex with unclear boundary between white matter and cortex in right occipital lobe (arrow). B. Axial 11C-methionine positron emission tomography/CT image showing slightly higher uptake of lesion than background (arrow).

References
1. Zhao C, Zhang Y, Wang J. A meta-analysis on the diagnostic performance of (18)F-FDG and (11)C-methionine PET for differentiating brain tumors. AJNR Am J Neuroradiol. 2014; 35:1058–1065.
2. Singhal T, Narayanan TK, Jain V, Mukherjee J, Mantil J. 11C-L-methionine positron emission tomography in the clinical management of cerebral gliomas. Mol Imaging Biol. 2008; 10:1–18.
3. Glaudemans AW, Enting RH, Heesters MA, Dierckx RA, van Rheenen RW, Walenkamp AM, et al. Value of 11C-methionine PET in imaging brain tumours and metastases. Eur J Nucl Med Mol Imaging. 2013; 40:615–635.
4. Nagata T, Tsuyuguchi N, Uda T, Terakawa Y, Takami T, Ohata K. Examination of 11C-methionine metabolism by the standardized uptake value in the normal brain of children. J Nucl Med. 2011; 52:201–205.
5. Lindholm P, Leskinen-Kallio S, Kirvelä O, Någren K, Lehikoinen P, Pulkki K, et al. Head and neck cancer: effect of food ingestion on uptake of C-11 methionine. Radiology. 1994; 190:863–867.
6. Torii K, Tsuyuguchi N, Kawabe J, Sunada I, Hara M, Shiomi S. Correlation of amino-acid uptake using methionine PET and histological classifications in various gliomas. Ann Nucl Med. 2005; 19:677–683.
7. Tomura N, Ito Y, Matsuoka H, Saginoya T, Numazawa SI, Mizuno Y, et al. PET findings of intramedullary tumors of the spinal cord using [18F] FDG and [11C] methionine. AJNR Am J Neuroradiol. 2013; 34:1278–1283.
8. Kawase Y, Yamamoto Y, Kameyama R, Kawai N, Kudomi N, Nishiyama Y. Comparison of 11C-methionine PET and 18F-FDG PET in patients with primary central nervous system lymphoma. Mol Imaging Biol. 2011; 13:1284–1289.
9. Okochi Y, Nihashi T, Fujii M, Kato K, Okada Y, Ando Y, et al. Clinical use of (11)C-methionine and (18)F-FDG-PET for germinoma in central nervous system. Ann Nucl Med. 2014; 28:94–102.
10. Phi JH, Paeng JC, Lee HS, Wang KC, Cho BK, Lee JY, et al. Evaluation of focal cortical dysplasia and mixed neuronal and glial tumors in pediatric epilepsy patients using 18F-FDG and 11C-methionine pet. J Nucl Med. 2010; 51:728–734.
11. Arita H, Kinoshita M, Okita Y, Hirayama R, Watabe T, Ishohashi K, et al. Clinical characteristics of meningiomas assessed by 11C-methionine and 18F-fluorodeoxyglucose positron-emission tomography. J Neurooncol. 2012; 107:379–386.
12. Takao H, Momose T, Ohtomo K. Methionine and glucose metabolism of central neurocytoma: a PET study. Clin Nucl Med. 2004; 29:838–839.
13. Nakagawa M, Kuwabara Y, Sasaki M, Koga H, Chen T, Kaneko O, et al. 11C-methionine uptake in cerebrovascular disease: a comparison with 18F-fDG PET and 99mTc-HMPAO SPECT. Ann Nucl Med. 2002; 16:207–211.
14. Harada Y, Hirata K, Kobayashi H, Usui R, Shiga T, Terae S, et al. A pitfall of C-11 methionine PET: cerebral venous infarction mimicked a glioma. Clin Nucl Med. 2012; 37:110–111.
15. O'Doherty MJ, Barrington SF, Campbell M, Lowe J, Bradbeer CS. PET scanning and the human immunodeficiency virus-positive patient. J Nucl Med. 1997; 38:1575–1583.
16. Sasaki M, Kuwabara Y, Yoshida T, Fukumura T, Morioka T, Nishio S, et al. Carbon-11-methionine PET in focal cortical dysplasia: a comparison with fluorine-18-FDG PET and technetium-99m-ECD SPECT. J Nucl Med. 1998; 39:974–977.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download