INTRODUCTION
Great saphenous vein (GSV) reflux is the most 369common underlying cause of significant varicose veins. Saphenopopliteal incompetence and small saphenous vein (SSV) reflux, although less common than GSV reflux, may result in symptoms of equivalent severity (
1234). It has traditionally been treated by open saphenopopliteal junction (SPJ) ligation with or without SSV stripping; however, the surgical method for incompetent SSV is more challenging and associated with more complications than for the GSV (
56). In fact, this surgery could lead to a high incidence of recurrence of up to 52% at 3 years, and it is frequently associated with neurovascular injury (
37).
Endovenous laser ablation (EVLA) is a relatively new, minimally invasive technique that was primarily developed to treat varicose veins due to saphenofemoral junction and GSV reflux with high success rates of 88–100% (
8). EVLA is certainly a more effective treatment modality for SPJ and SSV reflux than surgery. It has been developed as an alternative to surgery in an attempt to reduce morbidity and improve recovery time following varicose vein surgery (
3).
However, the operator could face a technical issue while performing EVLA for an incompetent SSV. The visualization of the laser-tip on the ultrasonographic image may be difficult, and in such cases, laser ablation cannot be performed successfully (
9). Another study reported the same difficulty while performing the EVLA for SSV, and in a total of nine cases, the precise location of the laser fiber around the SPJ could not be visualized and verified (
10). In addition, the caliber of the SSV is smaller than the GSV, and although prominent reflux is observed on a duplex ultrasound, appropriate venous access in the SSV for EVLA tends to remain difficult, inevitably resulting in multiple punctures.
Therefore, we attempted via the SSV 2–3 cm below the SPJ for retrograde EVLA to avoid multiple punctures in the SSV and reduce the time and effort needed to detect the laser tip in the SPJ. The aim of this study was to evaluate the safety and efficacy of this technique and compare it with the conventional antegrade EVLA for incompetent SSV.
Go to :

MATERIALS AND METHODS
Patients
From October 2009 to April 2014, among patients with varicose veins (C2–6) in unilateral or bilateral lower limbs who visited vascular outpatient departments, and those who were diagnosed as only having SSV reflux on duplex ultrasound were enrolled. Reflux was defined as reverse flow in the SSV for more than 0.5 seconds after releasing calf compression while standing.
Patients younger than 18 years and those with nonpalpable pedal pulses, deep vein thrombosis, a history of surgery for varicose veins, inability to ambulate, generally poor health, pregnancy, nursing or planning a pregnancy at some time during the course of treatment were excluded from the study (
10). Written informed consent was obtained on describing predicted results and potential complications of the technique. The Institutional Review Board approved the study and retrospective review of this data.
Procedure
The patient was placed in a prone position on the table in the treatment room, and was draped in the usual sterile manner from posterior mid-thigh to ankle. The SSV was cannulated via two approaches using a 22-gauge needle under ultrasound-guidance. One method involved puncturing the SSV cranially at mid-calf; these patients were classified into the antegrade group (AG). If the antegrade puncture into the SSV failed twice, the other approach for puncture was selected that involved puncturing the SSV toward the ankle, 2–3 cm distant from the SPJ, which could be accessed successfully under ultrasound-guidance. The patients who underwent this approach were defined as the retrograde group (RG) (
Fig. 1). Once the SSV was successfully punctured, a 0.018-inch guide wire was inserted into the needle and a 4 Fr or 5 Fr microsheath was introduced into the puncture site. Thereafter, a 0.035-inch guide wire was advanced in an antegrade or retrograde approach beyond the SPJ into the popliteal vein (in the AG) or beyond the SSV at the distal calf (in the RG) under ultrasound guidance. The guiding catheter was advanced over the guide wire and a sterile laser fiber was inserted into the catheter. The laser fibers were placed within 2 cm of the SPJ (in the AG) or the SSV at mid-calf (in the RG) under ultrasound or fluoroscopy. In the RG, the laser fiber was not placed over the mid-calf to reduce the risk of paresthesia by thermal damage to the nerve.
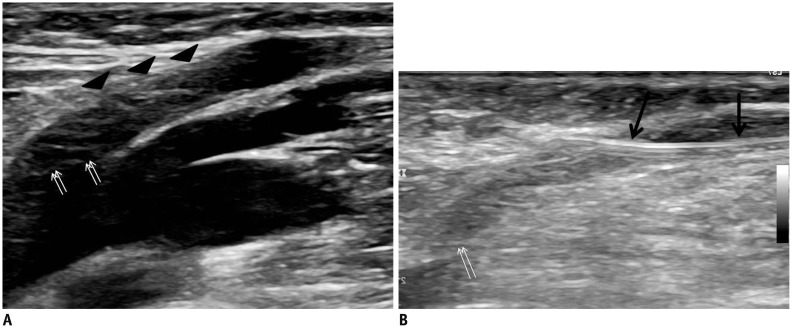 | Fig. 1
Retrograde access through SSV 2–3 cm distant from saphenopopliteal junction.
A. Ultrasound shows saphenopopliteal junction (arrows) and SSV (arrowheads). B. Ultrasound-guided puncture was performed for successful retrograde access (arrows) and saphenopopliteal junction (double arrows). SSV = small saphenous vein

|
Using ultrasound-guidance and a percutaneous needle, a tumescent solution consisting of 50–150 mL of 0.05% lidocaine was delivered along the course of the SSV within the perivenous space by using a 25-gauge needle. Tumescent fluid was injected around the SSV to ensure effective local anesthesia, compress and reduce the SSV diameter, provide vein wall apposition around the fiber tip, and minimize the possibility of heat-related damage to the adjacent tissue. The aiming beam of the tip of the laser fiber was visualized, and the laser fiber was pulled back through the skin.
After the procedure, the patient was discharged, and a class II full-thigh graduated support stocking was worn for at least 1 month at all times except during sleep or showering. Patients were prescribed analgesics for 3–7 days.
Follow-Up and Assessment
Patients were evaluated at the outpatient department at 1 week, and at 1, 3, 6, and 12 months, and annual follow-up and duplex ultrasound was performed. Postoperative pain scores (10 cm visual analogue scale [VAS]) were elicited at 1 week after EVLA and recorded. Bruising was measured by the investigator on a scale ranging from 0 (no bruise) to 5 (bruise over the entire segment and extension above or below the treatment segment). A bruise of less than 25% of the treated area was considered as 1; 25–50% as 2; 50–75% as 3; and 75–100% as 4 (
11). Sclerotherapy was performed for any remaining varicose veins. The incidences of adverse procedural sequelae, such as deep vein thrombosis, paresthesia, and infection, were also recorded.
The technical success of EVLA for the incompetent SSV was defined as successful access of the SSV, adequate placement of the endovenous laser fiber, and successful ablation of the targeted SSV. Successful closure of the targeted SSV on follow-up ultrasound was considered as clinical success. Duplex ultrasound criteria for successful closure included a non-compressible vein and no blood flow within the ablated SSV. The Student t test was used for quantitative value analysis such as pain, bruising, and linear endovenous energy density (LEED). Fisher's exact test was used for proportions. The closure rates were estimated using the Kaplan-Meier method, and differences between the two groups were assessed using the log-rank test. A p value < 0.05 was considered statistically significant.
Go to :

RESULTS
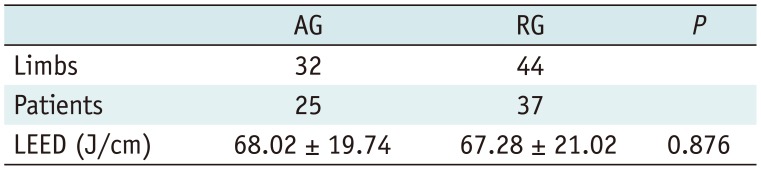
A total of 62 patients and 76 limbs were included: 25 patients and 32 limbs were managed using antegrade access and 37 patients and 44 limbs underwent EVLA after retrograde access. Technical success in venous access into the SSV, adequate placement of the laser fiber at the target vein, and complete ablation of the SSV were seen in all limbs in RG (100%). The 1470 nm endovenous laser with a bare tip fiber was used in all limbs. The LEED was 68.02 ± 19.74 in the AG and 67.28 ± 21.02 in the RG, without significant difference between the groups (
p = 0.876) (
Table 1).
Table 1
Summary of Results for AG and RG
|
AG |
RG |
P
|
|
Limbs |
32 |
44 |
|
|
Patients |
25 |
37 |
|
|
LEED (J/cm) |
68.02 ± 19.74 |
67.28 ± 21.02 |
0.876 |

In the AG, all limbs (100%) at 1-week follow-up showed complete closure of the treated saphenous vein (SV). Continued closure of the treated SSV was seen in 31 of 32 limbs (96.9%) at 1-month follow-up. All limbs (100%) showed complete closure at 3-month and 12-month follow-up, while 30 of 32 limbs (93.6%) had complete closure at 6-month follow-up. In the RG, all limbs at the 1-week and 1-month follow-up (100%) showed complete closure and 1 limb at 3-month and 2 limbs at 6-month follow-up (closure rate of 97.7% and 95.5%, respectively) showed recanalization. No significant difference was found in the closure rates of the SV treated by EVLA between the two groups (
p = 0.685) (
Fig. 2).
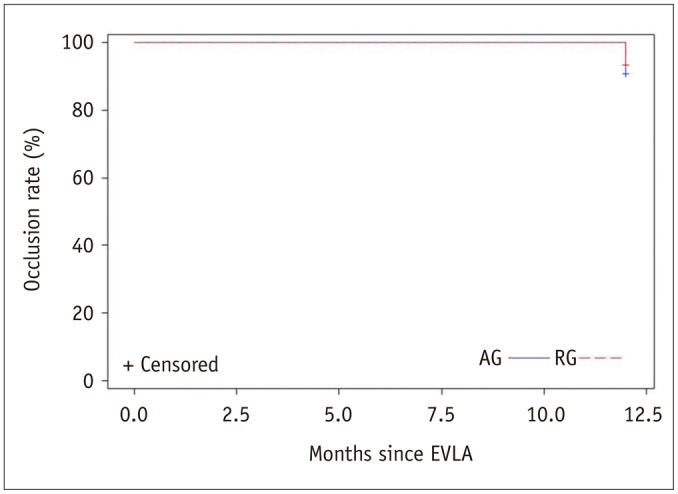 | Fig. 2Kaplan-Meier survival curve analysis of occlusion rate of incompetent small saphenous vein after endovenous laser ablation (EVLA) through antegrade group (AG) or retrograde group (RG) approach.
|
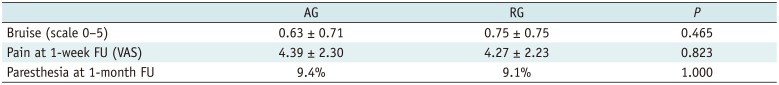
Three frequent complications including bruising, pain, and paresthesia, were noted. Bruise was determined by a scale, with mean score of 0.63 ± 0.71 in the AG and 0.75 ± 0.75 in the RG. These resolved completely in all followed-up limbs by one month. No statistically significant difference was found (
p = 0.465) (
Table 2). With reference to pain over the treatment site, the mean VAS scores were 4.39 ± 2.30 and 4.27 ± 2.23 at 1-week follow-up. Differences in pain levels did not reach statistical significance (
p = 0.823) (
Table 2). Paresthesia was detected in 3 (9.4%) limbs in the AG and 4 (9.1%) limbs in the RG at 1-month follow-up, without significant difference (
p = 1.000) (
Table 2). This symptom completely resolved at the next follow-up visit.
Table 2
Summary of Complications for AG and RG
|
AG |
RG |
P
|
|
Bruise (scale 0–5) |
0.63 ± 0.71 |
0.75 ± 0.75 |
0.465 |
|
Pain at 1-week FU (VAS) |
4.39 ± 2.30 |
4.27 ± 2.23 |
0.823 |
|
Paresthesia at 1-month FU |
9.4% |
9.1% |
1.000 |

Three limbs in the RG had tenderness at the puncture site in 3 patients lasting for a month (12%). This symptom completely resolved at 3-month follow-up. No significant complications occurred, such as skin burns, skin necrosis, pulmonary embolism, and deep vein thrombosis.
Go to :

DISCUSSION
Endovascular treatment of incompetent saphenous veins using radiofrequency or laser ablation has become the treatment of choice for lower-extremity venous insufficiency (
121314). Endovenous ablation is performed percutaneously, via an antegrade puncture into the lower saphenous vein under ultrasound-guidance. However, antegrade access into the saphenous vein under ultrasound-guidance is not feasible at all times. Small vessel diameter and vasospasm commonly make antegrade access difficult. Perosi et al. (
12) reported that the rationale for selection of retrograde access were small caliber of the GSV, vasospasm during access, previous, incomplete vein ablation, skin disease in the distal leg, or tortuous proximal GSV anatomy.
If antegrade percutaneous access fails, patients may be subjected to delays in treatment, multiple treatment attempts, and/or venous cutdown (
11). Therefore, an alternate approach to access incompetent saphenous veins should be considered to reduce the procedure time, increase the technical success rate of the procedure, and make the patients comfortable. Perosi et al. (
12) attempted to access and ablate incompetent GSVs retrogradely under fluoroscopy-guidance, when ultrasound-guided antegrade access during endovenous lower-extremity vein ablation was problematic. The technical success rate for retrograde access and subsequent ablation was 100% and no procedural complications occurred. Therefore, the authors concluded that the fluoroscopic retrograde approach can be used to treat the incompetent GSV when traditional antegrade access is not feasible. Park et al. (
10) also reported the alternative retrograde access route from the groin area for the 980-nm EVLA in case of an incompetent GSV; although they did not elucidate the number of cases with retrograde EVLAs for refluxing GSVs, a 99.7–100% successful ablation was reported during follow-up.
The inability to percutaneously access the SSV may be more common than that for the GSV. This can be due to the smaller caliber of the SSV for percutaneous access than the GSV, since the access into the GSV for endovascular treatment is obtained at the level of the knee and percutaneous puncture into the SSV tends to be performed at the posterior mid-calf. Therefore, operators face technical challenges when they attempt to access the SSV antegradely under ultrasound-guidance. We also failed to obtain antegrade access in 45 limbs, which developed vasospasm or had small diameters, making it difficult to obtain successful antegrade access to the SSV at mid-calf. Venous perforation or spasm, which occurs after inappropriate puncture at the access site of the SSV, prevents puncture at the same or adjacent site; thus, selection of an area higher up on the SSV than the initial puncture site is required during the next trial. At times, however, the distance between the puncture site selected after several failed attempts and the SPJ is too close, shortening the length of the SSV to be ablated; in addition, the operator may not be able to locate the dilated SSV for puncture under ultrasound-guidance. To obtain sufficient length of the proximal SSV to be punctured after failed attempts, some attempt puncturing the more distal SSV rather than the mid-calf. However, in some studies, temporary paresthesia is reported in as much as 40% of limbs following EVLA for the SSV (
2), and puncturing the SSV at the most distal point can increase postoperative nerve injury due to the close relationship between the SSV and the sural nerve at the level of the ankle. Doganci et al. (
3) concluded that puncturing the SSV at the mid-calf may decrease postoperative paresthesia. Therefore, we selected a different access route than the distal SSV to mid-calf as the initial puncture site.
The SSV 2–3 cm distant from the SPJ, which was selected as an access route, is larger than the SSV at the mid-calf. It is also located superficially and enveloped by the surrounding fascia. For these reasons, successful access is easy to obtain, and the laser fiber may be readily advanced into the SSV. Perosi et al. (
12) used the retrograde technique to treat GSV incompetence in a total of 38 legs in 33 patients, in whom the antegrade access failed. In the present study, we obtained 100% technical success of EVLA using a retrograde access. The retrograde technique for the SSV is likely to be easier than for the GSV, because the SSV is shorter and has a straighter course than the GSV.
Comparing the two groups (AG and RG), similar LEED was supplied during the EVLA in both groups. No significant difference was found between the two groups in terms of the closure rate for the SSV after EVLA, postoperative pain, and complications including bruising and paresthesia. Three limbs in the RG had tenderness at the puncture site lasting for one month, but these were the 1st, 2nd, and 4th patients who were treated by the retrograde approach. The prolonged tenderness was attributed to insufficient tumescent anesthesia around the puncture site. After supplying more tumescent anesthesia in succeeding patients, this complication did not recur. Park et al. (
15) reported the EVLA for SSV using a 980-nm laser through antegrade fashion and recanalization was 4% by 3 years follow-up. Gibson et al. (
16) reported 4% recanalization at 3 month follow-up using 980-nm laser and antegrade approach. The present data and comparison with other studies suggest that the retrograde EVLA for SSV does not show inferior results, as compared to the antegrade approach.
The present study has some limitations and shortcomings. First, we did not consider the anatomical variation of the SPJ at the site of attachment to the popliteal vein. Rashid et al. (
14) have shown that ligation for the SPJ is not achieved in 30% of the cases, even if the junction is marked preoperatively under ultrasound-guidance. In the present study, the retrograde puncture site was 2–3 cm distant from the SPJ and showed no anatomical variation. Since the SSV is slightly distant from the SPJ, it was considered suitable as the puncture site. Second, data collection over the 12-month follow-up period was not fully achieved. Third, we did not obtain the pain scale immediately after EVLA and at the 1-month follow-up because this was not a prospective study. Fourth, this is not a randomized controlled trial and may have significant selection bias.
In conclusion, the EVLA for the incompetent SSV using a retrograde approach is safe and effective and should be considered the alternative method if the antegrade access fails due to vasospasm or small SSV diameter.
Go to :






 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download