Abstract
Objective
This multi-center, randomized, double-blind, phase 3 trial was conducted to compare the safety and efficacy of contrast agents iohexol-380 and iohexol-350 for coronary CT angiography in healthy subjects.
Materials and Methods
Volunteers were randomized to receive 420 mgI/kg of either iohexol-350 or iohexol-380 using a flow rate of 4 mL/sec. All adverse events were recorded. Two blinded readers independently reviewed the CT images and conflicting results were resolved by a third reader. Luminal attenuations (ascending aorta, left main coronary artery, and left ventricle) in Hounsfield units (HUs) and image quality on a 4-point scale were calculated.
Results
A total of 225 subjects were given contrast media (115 with iohexol-380 and 110 with iohexol-350). There was no difference in number of adverse drug reactions between groups: 75 events in 56 (48.7%) of 115 subjects in the iohexol-380 group vs. 74 events in 51 (46.4%) of 110 subjects in the iohexol-350 group (p = 0.690). No severe adverse drug reactions were recorded. Neither group showed an increase in serum creatinine. Significant differences in mean density between the groups was found in the ascending aorta: 375.8 ± 71.4 HU with iohexol-380 vs. 356.3 ± 61.5 HU with iohexol-350 (p = 0.030). No significant differences in image quality scores between both groups were observed for all three anatomic evaluations (all, p > 0.05).
Computed tomography (CT) plays an important role in cardiovascular imaging (12). However, there are some limitations in obtaining diagnostic quality images in clinical practice. Sufficient vascular enhancement is a prerequisite for the accurate detection of coronary artery stenosis on coronary CT angiography (345). Achieving optimal coronary enhancement is particularly important for coronary CT angiography as coronary arteries are of small caliber, have a tortuous course, and diminution of blood flow is observed in the presence of stenosis or obstruction (6). Coronary artery attenuation is determined by the iodine administration rate (678); therefore, improved coronary enhancement can be achieved by either increasing the injection rate or by increasing the iodine concentration of contrast media. However, increased injection rate has the following limitations for clinical use of coronary CT angiography (39): 1) the injection rate used in clinical practice is already high, 4 to 5 mL/s; 2) larger needles, larger veins, and more set up time for the intravenous line would be necessary; and 3) the potential risk of contrast extravasation would increase. On the other hand, increasing the iodine concentration would be more feasible and without the above mentioned limitations. However, despite the several iodinated contrast media currently available on the market, high iodine concentration contrast media are very limited with only iopamidol-370 mgI/mL, iopromide-370 mgI/mL, and iomeprol-400 mgI/mL available (310). Currently, another new high iodine concentration, nonionic monomer, low osmolar contrast medium, 380 mgI/mL of iohexol is on trial. This multi-center, double-blind, randomized, comparative, phase 3 study was conducted to assess the safety and efficacy of the contrast agent, iohexol-380, for coronary CT angiography in comparison with iohexol-350 in healthy subjects.
This study was a multicenter, randomized, double-blind, active-controlled, parallel-group comparison conducted for a clinical trial 3rd phase according to the Guidelines of Good Clinical Practice with individual Institutional Review Board approval. Fourteen sites in Korea participated in the trial. All subjects provided written informed consent prior to enrollment.
Adult healthy volunteers (age, 20–65 years) weighing 50 to 90 kg were eligible for the study. Eligibility screening assessments included medical history taking, physical examination, pregnancy test, 12-lead electrocardiography examination, and laboratory evaluation. In addition to the absence of any known diseases, further specific exclusion criteria were as follows: history of hypersensitivity to iodinated contrast agents; known or suspected hyperthyroidism, pheochromocytoma, bronchial asthma, diabetes, myasthenia gravis, acute pancreatitis, vascular sclerosis, multiple myeloma or pulmonary hypertension; renal dysfunction; liver dysfunction; pregnant or lactating female; cardiac arrhythmia; or severe congestive heart failure (New York Heart Classification IV).
After enrollment in the study, volunteers were randomly assigned to receive either iohexol-350 (Bonorex 350; Centrial Medical Service, Seoul, Korea; 350 mg iodine per mL) or iohexol-380 (Bonorex 380; Centrial Medical Service, Seoul, Korea; 380 mg iodine per mL). Randomization sequence was created by a biostatistician with no clinical involvement in the trial using SAS statistical software (SAS system for Windows, version 9.2; SAS Institute, Cary, NC, USA), and stratified by center with a 1:1 allocation in a fixed block size of 4. Center-stratified randomization could allow for an equal allocation number of subjects to either group at each institute and thus avoid the effect from the use of different machines. Each pharmacy bulk package and iohexol container was identical in physical appearance and labeled with a random number. The treatment codes were concealed from the investigators enrolling participants, nurses, technicians, subjects, the independent readers, sponsor and their representatives. The treatment codes were delivered to the assistant investigator in sequentially numbered, opaque, sealed, and stapled envelopes, containing the information on a random number and the kind of contrast media. He/she measured the subjects' body weight and calculated the contrast volume to be administrated to each subject. These envelopes were kept in a locked cabinet, the sole responsibility of, and accessible to, the unblinded assistant investigator alone. The assistant investigator was instructed not to reveal the information on the subjects' body weight and treatment codes. He/she notified a random number to a pharmacist to deliver the contrast media labeled with the same number, and the calculated contrast volume to a technician who would administer contrast media to a subject.
Contrast media were administered through dual-head power injection into a peripheral vein of the right arm via an 18 G venous catheter. All volunteers in both groups were administered 420 mgI/kg of contrast media with the same flow rate of 4 mL/sec, followed by a 40-mL saline flush (0.9% NaCL solution). For example, a subject weighing 70 kg was given 77.4 mL of iohexol 380 mgI/mL (420 mgI/kg divided by 380 mgI/mL multiplied by 70 kg) and 84 mL of iohexol 350 mgI/mL (420 mgI/kg divided by 350 mgI/mL multiplied by 70 kg). Therefore, the total iodine amount was identical in both groups. Since volunteers weighing 50 to 90 kg were enrolled in the trial, volunteers in the iohexol-380 group were administered 55.3–99.5 mL of contrast medium with an iodine flux of 1.52 gI/s, corresponding to an injection duration of 14–25 seconds. The volunteers in the iohexol-350 group were administered 60–108 mL of contrast medium with an iodine flux of 1.4 gI/s, corresponding to an injection duration of 15–27 seconds.
Subjects underwent coronary CT angiography with five different 64 or 128-channel multidetector CT (MDCT) scanners: a 64-channel dual-source CT scanner (SOMATOM Definition; Siemens Medical Solutions, Forchheim, Germany), a 128-channel dual-source CT scanner (SOMATOM Definition Flash; Siemens Medical Solutions), two 64-channel MDCT scanners (Brilliance 64, Philips Medical Systems, Best, the Netherlands; GE LightSpeed VCTXT, General Electric Medical Systems, Milwaukee, WI, USA), and a 128-channel MDCT scanner (SOMATOM Definition AS Plus; Siemens Medical Solutions). Each institute followed a standardized protocol for the CT examination: craniocaudal direction; retrospective gating; bolus tracking in the descending aorta with a 100 Hounsfield units (HU) trigger and 8 second delay; tube voltage, 120 kVp; tube current-time product, adjustable; detector configuration, 64 x 0.6–0.625 mm, 32 x 0.6 or 64 x 0.6 mm in scanners; table speed, 0.2 pitch; gantry rotation time, 0.28–0.42 msec; matrix, 512 x 512; reconstruction slice thickness, 1 mm; reconstruction interval, 0.5–1 mm; and a standard algorithm reconstruction. Prospective sequential scanning was not allowed.
Figure 1 demonstrated an overview of study flowchart (Supplementary Table 1 in the online-only Data Supplement). In brief, laboratory tests including hematology and biochemistry were twice taken at least 14 days before and 24 hours after the CT examination: serum creatinine was also included (Supplementary Table 1 in the online-only Data Supplement). All subjects were monitored thrice by the research nurse at each institute at the visit on the day of CT examination and 24 hours after CT examination and on the phone 7 days after the CT examination for adverse events including a description of the event and the severity. The severity of adverse events was graded as mild, moderate or severe; mild, usually transient in nature and generally not interfering with normal activities; moderate, sufficiently discomforting to interfere with normal activities; and severe, incapacitating and prevents normal activities.
All CT images were interpreted by an independent review board, which consisted of three board-certified cardiovascular radiologists. Two readers (3-year and 9-year experiences in cardiovascular imaging) independently reviewed the CT images quantitatively and qualitatively. Any conflicting results between the two readers were finally resolved by the third reader (10-years of experience in cardiovascular imaging). The third reader also reviewed all CT images for technical adequacy and decided if the image sets were to be excluded for the efficacy assessment in case of severe motion, inadequate scan range, insufficient radiation, or other factors. All readers were fully blinded to patients allocation, as well as all clinical and other radiologic information.
Vessel densities (in HU) were independently measured by the two readers using circular regions of interest (ROIs) manually placed in the following regions: mid-portion of the ascending aorta for primary outcome measure, and mid-portion of left main coronary artery and the apical cavity of the left ventricle for secondary outcome measures (Fig. 2). ROIs were drawn as large as appropriate to avoid vessel walls, atheroma, or trabeculae. In subjects in whom there were differences of < 5 HU between the two readers, the average of the two measurements was used. In any subjects with a difference of > 5 HU, the measurements from the third reader were considered as the final results.
All evaluable coronary artery segments for three different anatomic regions including the ascending aorta, left main coronary artery, and the left ventricle were independently analyzed by two readers using the following 4-point scale: 1 (nondiagnostic), impaired image quality with poor definition of objects owing to inadequate enhancement and excessive image noise; 2 (adequate), evident limitations in vessel or left ventricular wall definition and in contrast resolution with suboptimal enhancement and moderate image noise; 3 (good), minimal limitations in vessel or left ventricular wall definition and in contrast resolution with sufficient enhancement and mild image noise; and 4 (excellent), excellent attenuation in the vessel lumen and clear vessel wall definition with barely perceived image noise (11). A decrease in image quality from motion and poor gating were not considered in the subjective assessment, as they are not likely to be affected by which contrast media was used (11). All conflicting results were reviewed and the final score was decided by the third reader.
Sample size was calculated using an nQuery Advisor program (nQuery Advisor, version 5.0, Statistical Solutions, Cork, Ireland) based on a t test to compare means between iohexol-380 and iohexol-350 groups. The minimum number of subjects required to have a power of 90% to discriminate between HU after the two regimens was 206 (103 in each group) and the maximum was 228 assuming a 10% attrition rate. Mean and standard deviation of the difference used were 25 HU and 55 HU, respectively, as described in a previous comparative study on iodixanol-320 and iohexol-350 by Tsai et al. (12). The Student t test for continuous variables and the χ2 test for categorical variable were used to compare the iohexol-380 and iohexol-350 groups. Estimated effect size and 95% confidence intervals were provided for the comparison of primary and secondary measures between two groups. All statistical tests were performed at a significance level of p < 0.05 using a statistical software package (SAS system for Windows, version 9.2).
Between July 20, 2012 and January 3, 2013, of the 276 subjects enrolled in the study, 48 were excluded after the screening test and 228 (82.6%) were randomly allocated to one of two contrast agent groups (115 in the iohexol-380 group and 113 in the iohexol-350 group) (Fig. 3). Among them, 4 subjects dropped out at the beginning of the study and 1 of them was administered contrast agents. Thus, 225 (115 in iohexol-380 and 110 in iohexol-350) were evaluated for safety assessment and 224 (114 in iohexol-380 and 110 in iohexol-350) for efficacy assessment. Demographic characteristics between the iohexol-380 and iohexol-350 groups showed no significant differences (Table 1).
The volunteers in the iohexol-380 group were administered an average of 72.8 mL (standard deviation, 11.3 mL; range, 55.8–97.6 mL) of contrast medium, corresponding to an injection duration of 14–24 seconds. The volunteers in the iohexol-350 group were administered an average of 79.3 mL (standard deviation, 12.5 mL; range, 70.1–106.8 mL) of contrast medium, corresponding to an injection duration of 17–27 seconds.
Mean values of white blood cell count, total protein, and albumin were significantly decreased after contrast administration in the iohexol-350 group although their changes were minimal (all p < 0.05), but differences in changes between the two groups were not significant (all, p > 0.05) (Supplementary Tables 2, 3 in the online-only Data Supplement). Mean values of eosinophil% and total bilirubin were significantly increased after contrast administration in both groups, without significant differences in changes between the two groups (all, p > 0.05) (Supplementary Tables 2, 3 in the online-only Data Supplement). No increase in serum creatinine above normal limits was found in either group (Supplementary Table 3 in the online-only Data Supplement).
The total number of adverse drug reactions was 150 events in 107 (47.6%) of 225 subjects: 76 events in 56 (48.7%) of 115 subjects in the iohexol-380 group vs. 74 events in 51 (46.4%) of 110 subjects in the iohexol-350 group (p = 0.690) (Table 2). Neither moderate or severe adverse drug reactions were recorded. The major adverse drug reactions reported in the iohexol-380 group were feeling hot in 46 (40.0%) subjects, followed by headaches in 11 (9.6%) subjects, and nausea in 6 (5.2%) subjects. Similarly, the major adverse drug reactions in the iohexol-350 group were also feeling hot in 46 (41.8%) subjects, followed by headaches in 15 (13.6%) subjects and urticaria in 5 (4.5%) subjects. There were no differences in frequency according to the types of adverse drug reactions between the iohexol-380 and iohexol-350 groups (all, p > 0.05).
Table 3 showed the results of density measurements at the three anatomic regions. According to the final assessment, mean density of the ascending aorta was significantly higher in the iohexol-380 group than in the iohexol-350 group (p = 0.030). However, mean densities of the left main coronary artery and left ventricle were not different between the two groups.
No differences in frequency according to image score category was found between the two readers and the final reports for all three anatomic evaluations (all, p > 0.05) (Table 4).
The principal findings of this study were first, iohexol-380, the new high concentration contrast medium on trial was as safe as the commercially available iohexol-350, with a similar incidence of mild adverse drug reactions and no incidence of severe adverse drug reactions; and second, the intravenous administration of iohexol-380 provided higher attenuation of the ascending aorta but similar attenuation of the coronary arteries, as compared to iohexol-350 with an identical amount of absolute iodine.
A number of studies have shown the effectiveness of contrast media with a high iodine concentration for vascular enhancement on CT angiography (5679131415). In two studies by Cademartiri et al. (9), the injection rate and volume were identical in all groups of contrast media with differing iodine concentrations; therefore, higher iodine delivery rates and higher total iodine amounts were used in groups with high iodine concentrations. They concluded that significantly higher attenuation in coronary arteries as well as thoracic aorta was achieved with the highest iodine concentration (913): comparison of iohexol-300, iodixanol-320, iohexol-350, iomeprol-350, and iomeprol-400 (13), and comparison between iopromide-370 and iomeprol-400 (9). Partly in agreement with these previous studies (91315), our results indicated higher attenuation in the ascending aorta but not in the coronary arteries with a contrast medium of high iodine concentration (iohexol-380), as compared with iohexol-350. This difference may have been due to the different injection protocols of the contrast media; identical iodine amounts of 420 mgI/kg with the same flow rate of 4 mL/sec were used in both groups, resulting in differing iodine delivery rates and injection volume. Vascular enhancement, however, is determined by the iodine delivery rate (iodine flux) (1617). Previous studies have shown conflicting results for vascular attenuations using identical iodine delivery rates in several contrast media with differing iodine concentrations (10151819). Rist et al. (15) used identical iodine delivery rates of 1 gI/s in both groups, and demonstrated that 63 mL of a contrast media with high iodine concentration (iomeprol-400) with a lower injection rate of 2.5 mL/s yielded equivalent coronary attention to that achieved after injection of 83 mL of iomeprol-300 at a rate of 3.3 mL/s. Another intra-individual comparison study of different contrast media concentrations (iopromide-300, iopromide-370, and iomeprol-400) in six pigs showed that the contrast medium with 300 mgI/mL provided improved contrast enhancement compared with a highly concentrated contrast medium in the thoracic aorta and pulmonary arteries, given identical iodine delivery rates and total iodine amount (18). It was assumed that lower viscosity and increased volume of iopromide-300 with a faster injection might enhance actual iodine delivery rates in the vessel, as compared to contrast media with higher concentrations (1820). However, this might not be applicable for coronary CT angiography, as the injection rates used in clinical practice are typically high at 4 to 5 mL/s and injection rates cannot be increased further.
We recognized some limitations in our study. First, an intra-individual comparison could have eliminated patient bias from hemodynamic differences, but were not performed in our study owing to ethical concerns. Second, the contrast media injection protocol used in our study was somewhat complicated. Most previous studies used one of the two following methods. One method is to keep the injection rate and volume constant, with differing iodine administration rates (iodine delivery rate). This method is typically used to assess the effectiveness of vascular enhancement of a contrast medium with high iodine concentration. The other method is to use identical iodine administration rates with identical total iodine amount, but differing injection rates. This method can be used to prove the equivalent power of vascular enhancement of a contrast medium with low concentrations, as compared to contrast media with higher concentrations. In our study, we used identical injection rates to assess the improved vascular enhancement of a contrast medium with high iodine concentrations, resulting in different iodine administration rates between both groups. However, we used identical total iodine amounts to assess the safety of the new contrast medium, iohexol-380, resulting in different volumes between groups. Therefore, the efficacy of high concentration contrast medium on vascular enhancement may have been reduced; indeed, we failed to see improved enhancement in the coronary arteries using iohexol-380. Lastly, different injection durations in each individual owing to administration of different contrast volumes may also have affected the timing of peak enhancement despite bolus-tracking.
In conclusion, iohexol-380 provides improved enhancement of the ascending aorta and similar attenuation in the coronary arteries without any increase in adverse drug reactions, as compared with iohexol-350 using an identical amount of total iodine.
Figures and Tables
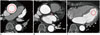 | Fig. 2Assessment of attenuation in three anatomical regions including ascending aorta (A), left main coronary artery (B), and left ventricular cavity (C).Circles indicate regions of interest placed at each region.
|
Table 1
Study Population Demographic Characteristics

Table 2
Adverse Drug Reactions

Table 3
Results of Objective Efficacy Assessment

Table 4
Image Quality Scores for Visualization of Ascending Aorta, Left Main Coronary Artery and Left Ventricle

Acknowledgments
Cental Medical Service was involved in the design and conduct of the study and provided contrast agents. Employees of the sponsor worked with the investigators to prepare the statistical analysis plan, but the efficacy analyses were performed by an independent radiologic committee of Seoul National University Hospital. The manuscript was prepared by Dr. Park, Dr. Lee and coauthors. Cental Medical Service was permitted to review the manuscript and suggest changes, but the final decision on content was exclusively retained by the authors.
References
1. Kim YJ, Yong HS, Kim SM, Kim JA, Yang DH, Hong YJ. Korean Society of Radiology. Korean Society of Cardiology. Korean guidelines for the appropriate use of cardiac CT. Korean J Radiol. 2015; 16:251–285.
2. Yoon YE, Lim TH. Current roles and future applications of cardiac CT: risk stratification of coronary artery disease. Korean J Radiol. 2014; 15:4–11.
3. Pugliese F. Which contrast agent for coronary ct angiography?. RAD Magazine. 2008. 10.
4. Cademartiri F, Mollet NR, Lemos PA, Saia F, Midiri M, de Feyter PJ, et al. Higher intracoronary attenuation improves diagnostic accuracy in MDCT coronary angiography. AJR Am J Roentgenol. 2006; 187:W430–W433.
5. Becker CR, Hong C, Knez A, Leber A, Bruening R, Schoepf UJ, et al. Optimal contrast application for cardiac 4-detector-row computed tomography. Invest Radiol. 2003; 38:690–694.
6. Fleischmann D. Use of high concentration contrast media: principles and rationale-vascular district. Eur J Radiol. 2003; 45:Suppl 1. S88–S93.
7. Herman S. Computed tomography contrast enhancement principles and the use of high-concentration contrast media. J Comput Assist Tomogr. 2004; 28:Suppl 1. S7–S11.
8. Bae KT. Peak contrast enhancement in CT and MR angiography: when does it occur and why? Pharmacokinetic study in a porcine model. Radiology. 2003; 227:809–881.
9. Cademartiri F, de Monye C, Pugliese F, Mollet NR, Runza G, van der Lugt A, et al. High iodine concentration contrast material for noninvasive multislice computed tomography coronary angiography: iopromide 370 versus iomeprol 400. Invest Radiol. 2006; 41:349–353.
10. Kim EY, Yeh DW, Choe YH, Lee WJ, Lim HK. Image quality and attenuation values of multidetector CT coronary angiography using high iodine-concentration contrast material: a comparison of the use of iopromide 370 and iomeprol 400. Acta Radiol. 2010; 51:982–989.
11. Lim J, Park EA, Lee W, Shim H, Chung JW. Image quality and radiation reduction of 320-row area detector CT coronary angiography with optimal tube voltage selection and an automatic exposure control system: comparison with body mass index-adapted protocol. Int J Cardiovasc Imaging. 2015; 31:Suppl 1. 23–30.
12. Tsai IC, Lee T, Tsai WL, Chen MC, Wu MJ, Lee WL, et al. Contrast enhancement in cardiac MDCT: comparison of iodixanol 320 versus iohexol 350. AJR Am J Roentgenol. 2008; 190:W47–W53.
13. Cademartiri F, Mollet NR, van der Lugt A, McFadden EP, Stijnen T, de Feyter PJ, et al. Intravenous contrast material administration at helical 16-detector row CT coronary angiography: effect of iodine concentration on vascular attenuation. Radiology. 2005; 236:661–665.
14. Brink JA. Use of high concentration contrast media (HCCM): principles and rationale--body CT. Eur J Radiol. 2003; 45:Suppl 1. S53–S58.
15. Rist C, Nikolaou K, Kirchin MA, van Gessel R, Bae KT, von Ziegler F, et al. Contrast bolus optimization for cardiac 16-slice computed tomography: comparison of contrast medium formulations containing 300 and 400 milligrams of iodine per milliliter. Invest Radiol. 2006; 41:460–467.
16. Bae KT, Heiken JP. Scan and contrast administration principles of MDCT. Eur Radiol. 2005; 15:Suppl 5. E46–E59.
17. Cademartiri F, van der Lugt A, Luccichenti G, Pavone P, Krestin GP. Parameters affecting bolus geometry in CTA: a review. J Comput Assist Tomogr. 2002; 26:598–607.
18. Behrendt FF, Pietsch H, Jost G, Sieber MA, Keil S, Plumhans C, et al. Intra-individual comparison of different contrast media concentrations (300mg, 370mg and 400mg iodine) in MDCT. Eur Radiol. 2010; 20:1644–1650.
19. Faggioni L, Neri E, Sbragia P, Pascale R, D'Errico L, Caramella D, et al. 80-kV pulmonary CT angiography with 40mL of iodinated contrast material in lean patients: comparison of vascular enhancement with iodixanol (320mg I/mL)and iomeprol (400mg I/mL). AJR Am J Roentgenol. 2012; 199:1220–1225.
20. Han JK, Kim AY, Lee KY, Seo JB, Kim TK, Choi BI, et al. Factors influencing vascular and hepatic enhancement at CT: experimental study on injection protocol using a canine model. J Comput Assist Tomogr. 2000; 24:400–406.
Supplementary Materials
The online-only Data Supplement is available with this article at http://dx.doi.org/10.3348/kjr.2016.17.3.330.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download