Abstract
Objective
To determine the patho-mechanism of pleural effusion or hydropneumothorax in Mycobacterium avium complex (MAC) lung disease through the computed tomographic (CT) findings.
Materials and Methods
We retrospectively collected data from 5 patients who had pleural fluid samples that were culture-positive for MAC between January 2001 and December 2013. The clinical findings were investigated and the radiological findings on chest CT were reviewed by 2 radiologists.
Results
The 5 patients were all male with a median age of 77 and all had underlying comorbid conditions. Pleural fluid analysis revealed a wide range of white blood cell counts (410–100690/µL). The causative microorganisms were determined as Mycobacterium avium and Mycobacterium intracellulare in 1 and 4 patients, respectively. Radiologically, the peripheral portion of the involved lung demonstrated fibro-bullous changes or cavitary lesions causing lung destruction, reflecting the chronic, insidious nature of MAC lung disease. All patients had broncho-pleural fistulas (BPFs) and pneumothorax was accompanied with pleural effusion.
Mycobacterium avium complex (MAC), consisting of 2 species of Mycobacterium avium (M. avium) and Mycobacterium intracellulare (M. intracellulare), is the most common etiologic organism of lung disease caused by nontuberculous mycobacteria (NTM) (12). The prevalence of MAC lung diseases is increasing worldwide (3456). The traditionally recognized clinical presentation of MAC lung disease is apical fibrocavitary disease. This type of disease usually develops in older males with a history of lung disease, such as previous pulmonary tuberculosis (TB) (7). MAC lung disease can also present with nodular infiltrates, frequently involving the right middle lobe and the lingular segment of the left upper lobe. This form of disease is termed nodular bronchiectasis and occurs predominantly in postmenopausal, non-smoking females (8910).
Although some of the clinical and radiographic features of NTM lung disease resemble those of pulmonary TB, NTM infection is rarely accompanied by pleural involvement (11). Pleural TB develops from pulmonary lesions that form a small subpleural TB focus. Mycobacterial antigens enter the pleural space and interact with T-cells previously sensitized to mycobacteria, resulting in acute inflammation and exudation caused by a delayed hypersensitivity reaction (1213). Chest computed tomography (CT) frequently shows that subpleural parenchymal lesions are associated with TB pleurisy (1415).
However, pleural disease is not often found and pleural effusion is rare in NTM lung disease (2). Christensen et al. (16) examined the initial radiographic appearance of 100 cases of MAC lung disease. Only 6% of cases had a defined edge of pleural thickening or fluid blunting of the lateral or posterior costophrenic sulcus. Lynch et al. (17) reported on the CT features of 55 cases of MAC lung disease and found that only 7% of cases had pleural calcification. There have been a few case reports on pleural effusion associated with MAC lung disease in the English literature (18192021). Here, we presented 5 cases of pleural hydropneumothorax associated with MAC lung disease, and suggest a possible mechanism for the development of pleural effusion in patients with MAC lung disease through the CT findings.
This study protocol was approved by the Institutional Review Board of Samsung Medical Center. Informed consent was waived due to the retrospective nature of this study. Using the mycobacterial laboratory database, we identified and reviewed the medical records of 6 consecutive patients of Samsung Medical Center (a 1961-bed referral hospital in Seoul, Korea) who had MAC culture-positive pleural fluid samples between January 2001 and December 2013. One patient who developed MAC culture-positive pleural effusion as a post-operative complication after resectional surgery for MAC lung disease was excluded. Consequently, 5 patients were ultimately included in the study.
During the study period, MAC species were identified using a polymerase chain reaction-restriction fragment length polymorphism method based on the rpoB gene, as previously described (222324). All patients fulfilled the diagnostic criteria set forth by the American Thoracic Society/Infectious Diseases Society of America in 2007 (2). Clinical data for 1 patient was published in a 2006 case report (20).
All data for the patients, which included age, sex, past history of pulmonary TB, underlying comorbid conditions and gross findings of pleural fluid were drawn from patient charts. Blood laboratory findings, such as the levels of total protein, lactate dehydrogenase (LDH), glucose, and white blood cell (WBC) count were reviewed. Pleural fluid examination included WBC count with the proportion of neutrophils and lymphocytes, the levels of total protein, LDH, glucose and adenosine deaminase (ADA). Microbiological findings, such as the causative microorganisms, were also reviewed.
All CT examinations were performed using various helical CT scanners (Light SpeedVCT and Discovery CT750 HD, GE Healthcare, Chalfont St. Giles, England; Brilliance 40, Philips, Best, the Netherlands; Aquilion, Toshiba, Tokyo, Japan). CT scans (114–210 mA, 120 kVp, beam width of 10–20 mm, beam pitch of 1.375–1.5) were obtained from the lung apices to the level of the middle portion of both kidneys. The image data were reconstructed with a section thickness of 2.5–5 mm using a bone algorithm. The reconstructed images were then interfaced directly with a picture archiving and communication system (Centricity 2.0; GE Healthcare, Mt. Prospect, IL, USA), which displayed all image data on 2 monitors (1536 × 2048 matrix, 8-bit viewable gray scale, and 60-ft-Lambert luminescence). Both mediastinal (width, 400 Hounsfield unit [HU]; level, 20 HU) and lung (width, 1500 HU; level, -700 HU) window images were viewed on these monitors.
Two chest radiologists, blinded to all clinical information other than that the patients had MAC culture-positive pleural fluid, jointly assessed the CT images and decisions on CT findings were reached by consensus (with 5 and 22 years of experience in chest CT interpretation, respectively). The presence of all parenchymal abnormality patterns in each lobe (6 lobes: right upper lobe, right middle lobe, right lower lobe, upper division of left upper lobe, lingular division of left upper lobe, and left lower lobe) was recorded. The assessed patterns of parenchymal abnormalities included: nodule (10–30 mm in diameter), tree-in-bud opacity (small centrilobular nodules < 10 mm in diameter and branching nodular structures), consolidation (lobular, segmental, or peribronchial), cavities (single or multiple regardless of their size or wall thickness), bronchiectasis, and lobar volume decrease. Lobular consolidation was defined as a consolidative lesion of 0.5–3.0 cm in size, polygonal in shape, and with a subpleural location. Segmental consolidation was defined as a larger pleural-based consolidative lesion with a polygonal appearance rather than lobular consolidation. Peribronchial consolidation was defined as a consolidative lesion distributed along the bronchovascular bundles. A lobar volume decrease was regarded as present when a lobe contained crowded bronchovascular bundles, peribronchial reticulation, and bronchial dilatation with a distorted and displaced fissure (25). The extent of the parenchymal abnormality was estimated by counting the number of lobes involved. The laterality (unilateral or bilateral) of lung lesions was also analyzed. The site (right or left), presence of a broncho-pleural fistula (BPF) and pleural thickening were assessed for hydropneumothorax. BPFs are direct communications between the pleural space and the bronchial tree or lung parenchyma (26). Additionally, the time interval between the presence of the hydropneumothorax on the CT scan and the initial parenchymal NTM diagnosis was recorded.
The clinical findings from the 5 MAC culture-positive patients with pleural effusions were shown in Table 1. The median age was 77 years (range 56–86 years) and all were male. Three patients had a history of pulmonary TB treatment and all patients had underlying comorbid diseases that included chronic obstructive pulmonary disease, diabetes mellitus, rheumatoid arthritis, liver cirrhosis and prostate cancer. In blood laboratory tests, the WBC counts were in the normal range, and the nutritional status, reflected by the measured protein level, was also in the normal range in all patients. The level of fasting glucose was mildly elevated in 1 patient. With respect to the microbiological findings in pleural fluids, the causative microorganisms consisted of M. avium in 1 patient and M. intracellulare in 4 patients. Pleural fluid analysis revealed variable WBC counts (410–100690/µL) with various proportions of neutrophils (15–100%). The levels of LDH and ADA were elevated (56.3–206.6 IU/L) in the 3 patients from whom samples were available.
Among the 5 patients who underwent chest CT, all had abnormal parenchymal lesions representative of MAC lung disease. Table 2 presented a summary of the abnormal chest CT findings in the 5 patients. The tree-in-bud pattern was seen in 3 patients (60%) and segmental or peribronchial consolidation and bronchiectasis with lobar or segmental volume loss causing lung destruction was present in all patients (100%). Cavitary lesions were present in 4 patients (80%). Bilateral involvement of lung lesions was observed in 3 patients (60%). Hydropneumothorax accompanied by pleural thickening was observed in all patients. Two radiologists reviewed the findings with respect to the presentation of pleural effusions as hydropneumothorax, particularly for the presence of a BPF. The finding was that all patients had ≥ 1 BPFs related to hydropneumothorax (Figs. 1, 2). The time interval between the presence of a hydropneumothorax on CT and the initial parenchymal NTM diagnosis in 3 of the patients ranged from 36–89 months; however, in the remaining 2 patients, the hydropneumothorax was present at the time of initial diagnosis of MAC lung disease.
The significant findings of this study were as follows: 1) all patients with complications involving hydropneumothorax were male and had underlying comorbid disease; 2) most pleural fluid samples had higher leukocyte counts with predominant neutrophils and high LDH levels suggestive of empyema; 3) most patients had extensive pulmonary lesions and multiple cavities or bronchiectasis due to MAC lung disease; 4) all patients had ≥ 1 BPFs associated with hydropneumothorax in CT; 5) these MAC lung disease with hydropneumothorax are extremely rare in our 13-year single-tertiary center experience. Based on the results of this study, it is possible that the development of hydropneumothorax with BPFs is related to the coexistence of MAC lung disease.
Mycobacterial pleurisy is a common etiology of lymphocyte-rich pleural effusion, and is commonly caused by Mycobacterium tuberculosis (27). In contrast, NTM pleurisy is uncommon, with only a few cases based on diverse diagnostic criteria reported in the literature (18192021). With the increasing prevalence of NTM disease (3456), NTM pleurisy has become a clinical concern. Thus, in patients with MAC lung disease who present with pleural effusion, differentiation between NTM pleurisy and other superimposed infection (e.g., TB pleurisy or bacterial exudate) is important. During the 13-year study in this hospital, MAC was isolated from pleural effusion in only 5 patients with MAC lung disease. In this study, the patients with MAC pleurisy had higher leukocyte counts in their pleural effusions than did patients with known TB pleurisy, possibly due to differences in the nature of the micro-organisms. Previous studies on MAC pleurisy have reported neutrophil predominant pleural effusion and high LDH levels (2028).
Although, the pathway leading from NTM infection to pleural empyema is currently unclear, 2 theories are proposed (29); first, the development of the empyema from the lung infection and second, the development of the pleural effusion after minor trauma. The CT imaging findings in this study clearly demonstrated BPFs and loculated hydropneumothorax. These findings suggested that in our cases, the pleural effusion resulted from pulmonary parenchymal infection through BPFs, as previously suggested (29). While BPFs associated with TB usually follow a surgical procedure, they can also occur spontaneously (3031). Our study suggested that the fibro-bullous or fibro-cavitary changes caused by previous MAC lung disease play a potential role in the development of BPFs and subsequent pleural effusion due to MAC infection. This assumption was supported by 3 of our cases (case no. 2, 4, and 5), which showed the slowly progressive of nature of MAC lung disease (1032), resulting in hydropneumothorax months or years after the initial diagnosis. Therefore, BPFs can be a sign for distinguishing from other secondary exudates.
In a recent Taiwanese study (33) on 35 patients with NTM pleurisy, only 3 patients had pulmonary involvement (3/35, 8.6%) unlike our study finding, possibly due to more severe underlying comorbid condition (e.g., malignancy or immune dysfunction) and younger median age of the population (51.2 years) than that of our study (77 years). Furthermore, non-MAC NTM species were isolated in 19 patients in the Taiwanese study; whereas, only MAC species were found in our study. However, the possible pathogenic mechanism of NTM pleurisy in Taiwan is unclear and may differ from our assumption. Patient cohorts with different local epidemiologic features in Taiwan must be investigated in order to better understand the pathogenesis of NTM pleurisy.
Previous studies have indicated that malnutrition, impaired cellular immunity, apparently abnormal microvascular circulation due to diabetes mellitus (18) and surgical treatment (34) may enhance the development of pleuritis in patients with underlying MAC infections. In our study, the patients also had comorbid conditions. Not all, but some of these cases may have been affected by these comorbid diseases, resulting in underlying fibro-bullous or fibro-cavitary changes, which may provide a possible explanation for the progression of MAC disease and ultimately the development of pleural empyema.
Our study had several limitations. First, as this was a retrospective study, the number of patients with MAC infections may have been underestimated. For mycobacterial culture, liquid culture media reportedly result in higher yields and faster results than solid media. At our institution, a liquid culture-based method (mycobacterial growth indicator tube [MGIT]; Becton-Dickinson and Co., Sparks, MD, USA) was introduced in January, 2009 (35). Therefore, patients with pleural effusion who had culture-positive MAC may have had more advanced MAC lung disease, particularly in the early study period. Second, due to the small number of cases, no firm conclusions can be drawn, and a large-scale prospective study is needed.
In conclusion, all 5 patients with underlying MAC lung disease and culture-positive MAC pleural effusion had fistulous communication between the pulmonary NTM lesions and the pleural space. Thus, in patients with underlying MAC lung disease who present with pleural effusion, the presence of BPFs and pleural air on CT imaging is indicative that the spread of MAC infection is the cause of the effusion.
Figures and Tables
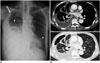 | Fig. 1Chest radiograph and CT images obtained from 86-year-old man with pulmonary disease and hydropneumothorax caused by Mycobacterium avium (Case 4).
A. Chest radiography shows hydropneumothorax (arrow) in right hemithorax. Small cavitary or non-cavitary nodules and branching nodular structures (tree-in-bud pattern) (arrowheads) are seen in both lungs. B. CT images with lung and mediastinal window setting demonstrate hydropneumothorax and enhancing pleural thickening suggesting pleural empyema. Note broncho-pleural fistulas (arrow) that developed from prior cavitary lesion (not shown) in same area.
|
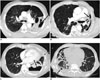 | Fig. 2CT images showing broncho-pleural fistula (arrows) in each case with hydropneumothorax associated with Mycobacterium avium complex lung disease (A–D, Cases 1–3, and 5, respectively). |
Table 1
Clinical Characteristics of Patients with MAC Lung Disease with Pleural Involvement

ADA = adenosine deaminase, COPD = chronic obstructive pulmonary disease, LDH = lactate dehydrogenase, M. avium = Mycobacterium avium, M. intracellulare = Mycobacterium intracellulare, MAC = Mycobacterium avium complex, NA = not available due to high viscosity of pleural fluid, WBC = white blood cell
Table 2
CT Findings from Patients with MAC Lung Disease with Pleural Involvement

References
1. Daley CL, Griffith DE. Pulmonary non-tuberculous mycobacterial infections. Int J Tuberc Lung Dis. 2010; 14:665–671.
2. Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007; 175:367–416.
3. Hoefsloot W, van Ingen J, Andrejak C, Angeby K, Bauriaud R, Bemer P, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J. 2013; 42:1604–1613.
4. Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013; 34:87–94.
5. Koh WJ, Chang B, Jeong BH, Jeon K, Kim SY, Lee NY, et al. Increasing recovery of nontuberculous mycobacteria from respiratory specimens over a 10-year period in a tertiary referral hospital in South Korea. Tuberc Respir Dis (Seoul). 2013; 75:199–204.
6. Kwon YS, Koh WJ. Diagnosis of pulmonary tuberculosis and nontuberculous mycobacterial lung disease in Korea. Tuberc Respir Dis (Seoul). 2014; 77:1–5.
7. Aksamit TR. Mycobacterium avium complex pulmonary disease in patients with pre-existing lung disease. Clin Chest Med. 2002; 23:643–653.
8. Chung MJ, Lee KS, Koh WJ, Lee JH, Kim TS, Kwon OJ, et al. Thin-section CT findings of nontuberculous mycobacterial pulmonary diseases: comparison between Mycobacterium avium-intracellulare complex and Mycobacterium abscessus infection. J Korean Med Sci. 2005; 20:777–783.
9. Lee G, Kim HS, Lee KS, Koh WJ, Jeon K, Jeong BH, et al. Serial CT findings of nodular bronchiectatic Mycobacterium avium complex pulmonary disease with antibiotic treatment. AJR Am J Roentgenol. 2013; 201:764–772.
10. Lee G, Lee KS, Moon JW, Koh WJ, Jeong BH, Jeong YJ, et al. Nodular bronchiectatic Mycobacterium avium complex pulmonary disease. Natural course on serial computed tomographic scans. Ann Am Thorac Soc. 2013; 10:299–306.
11. Koh WJ, Yu CM, Suh GY, Chung MP, Kim H, Kwon OJ, et al. Pulmonary TB and NTM lung disease: comparison of characteristics in patients with AFB smear-positive sputum. Int J Tuberc Lung Dis. 2006; 10:1001–1007.
12. Jeon D. Tuberculous pleurisy: an update. Tuberc Respir Dis (Seoul). 2014; 76:153–159.
13. Light RW. Update on tuberculous pleural effusion. Respirology. 2010; 15:451–458.
14. Kim HJ, Lee HJ, Kwon SY, Yoon HI, Chung HS, Lee CT, et al. The prevalence of pulmonary parenchymal tuberculosis in patients with tuberculous pleuritis. Chest. 2006; 129:1253–1258.
15. Ko JM, Park HJ, Kim CH. Pulmonary changes of pleural TB: up-to-date CT imaging. Chest. 2014; 146:1604–1611.
16. Christensen EE, Dietz GW, Ahn CH, Chapman JS, Murry RC, Anderson J, et al. Initial roentgenographic manifestations of pulmonary Mycobacterium tuberculosis, M kansasii, and M intracellularis infections. Chest. 1981; 80:132–136.
17. Lynch DA, Simone PM, Fox MA, Bucher BL, Heinig MJ. CT features of pulmonary Mycobacterium avium complex infection. J Comput Assist Tomogr. 1995; 19:353–360.
18. Nagaia T, Akiyama M, Mita Y, Tomizawa T, Dobashi K, Mori M. Mycobacterium avium complex pleuritis accompanied by diabetes mellitus. Diabetes Res Clin Pract. 2000; 48:99–104.
19. Okada Y, Ichinose Y, Yamaguchi K, Kanazawa M, Yamasawa F, Kawashiro T. Mycobacterium avium-intracellulare pleuritis with massive pleural effusion. Eur Respir J. 1995; 8:1428–1429.
20. Park SU, Koh WJ, Kwon OJ, Park HY, Jun HJ, Joo EJ, et al. Acute pneumonia and empyema caused by Mycobacterium intracellulare. Intern Med. 2006; 45:1007–1010.
21. Yanagihara K, Tomono K, Sawai T, Miyazaki Y, Hirakata Y, Kadota J, et al. Mycobacterium avium complex pleuritis. Respiration. 2002; 69:547–549.
22. Jeong BH, Jeon K, Park HY, Kim SY, Lee KS, Huh HJ, et al. Intermittent antibiotic therapy for nodular bronchiectatic Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2015; 191:96–103.
23. Koh WJ, Jeong BH, Jeon K, Lee NY, Lee KS, Woo SY, et al. Clinical significance of the differentiation between Mycobacterium avium and Mycobacterium intracellulare in M avium complex lung disease. Chest. 2012; 142:1482–1488.
24. Koh WJ, Jeong BH, Jeon K, Lee SY, Shin SJ. Therapeutic drug monitoring in the treatment of Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. 2012; 186:797–802.
25. Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008; 246:697–722.
26. Stern EJ, Sun H, Haramati LB. Peripheral bronchopleural fistulas: CT imaging features. AJR Am J Roentgenol. 1996; 167:117–120.
27. Ferrer J. Pleural tuberculosis. Eur Respir J. 1997; 10:942–947.
28. Kakugawa T, Mukae H, Kajiki S, Tanaka A, Yamayoshi T, Inoue M, et al. Mycobacterium avium pleuritis in a non-immunocompromised patient. Intern Med. 2008; 47:1727–1731.
29. Kotani K, Hirose Y, Endo S, Yamamoto H, Makihara S. Surgical treatment of atypical Mycobacterium intracellulare infection with chronic empyema: a case report. J Thorac Cardiovasc Surg. 2005; 130:907–908.
30. Kim HY, Song KS, Goo JM, Lee JS, Lee KS, Lim TH. Thoracic sequelae and complications of tuberculosis. Radiographics. 2001; 21:839–858. discussion 859-860.
31. Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest. 2005; 128:3955–3965.
32. Kim SJ, Park J, Lee H, Lee YJ, Park JS, Cho YJ, et al. Risk factors for deterioration of nodular bronchiectatic Mycobacterium avium complex lung disease. Int J Tuberc Lung Dis. 2014; 18:730–736.
33. Shu CC, Lee LN, Wang JT, Chien YJ, Wang JY, Yu CJ. Taiwan Anti-Mycobacteria Investigation (TAMI) Group. Non-tuberculous mycobacterial pleurisy: an 8-year single-centre experience in Taiwan. Int J Tuberc Lung Dis. 2010; 14:635–641. 4 p following 641.
34. Kawamoto H, Yamagata M, Nakashima H, Kambe M, Okamoto N, Yamane K, et al. Development of a case of Mycobacterium avium complex disease from right pleural effusion. Nihon Kokyuki Gakkai Zasshi. 2000; 38:706–709.
35. Koh WJ, Ko Y, Kim CK, Park KS, Lee NY. Rapid diagnosis of tuberculosis and multidrug resistance using a MGIT 960 system. Ann Lab Med. 2012; 32:264–269.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download