Abstract
Sclerosing cholangitis is a spectrum of chronic progressive cholestatic liver disease characterized by inflammation, fibrosis, and stricture of the bile ducts, which can be classified as primary and secondary sclerosing cholangitis. Primary sclerosing cholangitis is a chronic progressive liver disease of unknown cause. On the other hand, secondary sclerosing cholangitis has identifiable causes that include immunoglobulin G4-related sclerosing disease, recurrent pyogenic cholangitis, ischemic cholangitis, acquired immunodeficiency syndrome-related cholangitis, and eosinophilic cholangitis. In this review, we suggest a systemic approach to the differential diagnosis of sclerosing cholangitis based on the clinical and laboratory findings, as well as the typical imaging features on computed tomography and magnetic resonance (MR) imaging with MR cholangiography. Familiarity with various etiologies of sclerosing cholangitis and awareness of their typical clinical and imaging findings are essential for an accurate diagnosis and appropriate management.
Sclerosing cholangitis includes a spectrum of chronic, variably progressive cholestatic liver disease characterized by inflammation, fibrosis, and stricture of the intrahepatic 38and extrahepatic bile ducts (1). Sclerosing cholangitis can be divided into primary sclerosing cholangitis (PSC) of unidentified etiology and secondary sclerosing cholangitis caused by various identifiable etiologies, including immunoglobulin G4-related sclerosing cholangitis (IgG4-SC), recurrent pyogenic cholangitis (RPC), ischemic cholangitis, acquired immunodeficiency syndrome (AIDS)-related cholangitis, and eosinophilic cholangitis. The diagnosis of PSC requires the exclusion of secondary causes of sclerosing cholangitis. Furthermore, contrary to PSC in which liver transplantation is recommended as a curative treatment option, secondary sclerosing cholangitis can respond favorably to treatment for related causes. Therefore, awareness of the imaging features of primary and secondary sclerosing cholangitis and the clinical setting are important for accurate diagnosis in patients with sclerosing cholangitis.
In this article, we describe the imaging findings of the various spectrums of sclerosing cholangitis with an emphasis on a systemic approach in differential diagnosis. We also discuss the clinical significance of and therapeutic options for treating sclerosing cholangitis.
Primary sclerosing cholangitis is a chronic, progressive liver disease with inflammation and fibrosis of the bile ducts of unidentified etiology, which finally progresses to biliary cirrhosis and portal hypertension (23).
The prevalence of PSC is approximately 10/100000 in Northern Europe (45) and the USA, while it is far less common in Southern Europe and Asia (67). PSC affects men twice as often as women, and generally young patients with an age of onset of 30-40 years. PSC has a strong association with inflammatory bowel diseases. Approximately 60 to 80% of patients with PSC present with inflammatory bowel disease of which, 87% have ulcerative colitis and 13% have Crohn's disease (89). The clinical presentation can vary, including cholestatic laboratory findings and nonspecific symptoms including right upper quadrant pain or jaundice.
Although the pathogenesis remains to be elucidated, many experts accept an autoimmune component (101112). A typical histopathologic feature of PSC is substantial periductal ("onion-skin") fibrosis with minimal inflammatory cells (Fig. 1). However, this finding is observed in < 20% of cases and may also be found in secondary sclerosing cholangitis (13). Therefore, a liver biopsy is not recommended for the diagnosis of PSC in patients with typical cholangiographic findings (14).
Diagnosis of PSC can be made by typical cholangiographic findings and the exclusion of secondary causes. Both the American Association for the Study of Liver Diseases and the European Association of the Study of the Liver guidelines recommend magnetic resonance (MR) cholangiography as the first option for cholangiography in cases of suspected PSC, and endoscopic cholangiography for non-diagnostic cases (1415). The typical MR cholangiographic features include diffuse, multifocal short segmental strictures and mild dilatation in the intrahepatic and extrahepatic bile ducts alternating with normal ducts, which sometimes produce "beaded" appearance (Figs. 2, 3). As the fibrosis progresses and strictures worsen, the peripheral bile ducts are obliterated and become poorly visualized on MR cholangiography showing a "pruned tree" appearance (Fig. 3) (16). Diverticular outpouching of bile ducts is another characteristic finding that occurs in up to 27% of the patients with PSC (Fig. 2) (17). Almost half of patients with PSC have some degree of mural irregularity causing a shaggy or nodular appearance of the bile ducts (18). PSC commonly involves both intrahepatic and extrahepatic ducts in 75% of patients, whereas involvement of only the extrahepatic bile duct is uncommon (10% of patients) and isolated involvement of the intrahepatic bile ducts is reported in 15% of patients (3). Bile duct stones are detected in 8% of these patients (16).
Abdominal ultrasonography (US) is generally non-diagnostic, although thickened or focal dilated bile ducts are observed in PSC patients. Computed tomography (CT) demonstrates alternating narrowing and dilatation of the bile ducts with contrast enhancement in PSC (19). CT and MR imaging also show associated parenchymal changes of the liver, as well as ductal changes in PSC. A rounded liver appearance is observed due to hypertrophy of the caudate lobe and atrophy of the left lateral and right posterior segments in PSC (9). In PSC, T2-weighted MR images show a wedge-shaped or reticular heterogeneous area of high-signal intensity with peripheral distribution (20).
Patients with PSC have a 10 to 15% risk of developing cholangiocarcinomas, and a 7 to 9% chance of a 10-year cumulative incidence of cholangiocarcinomas (1621). Diagnostic evaluation of suspected cholangiocarcinoma includes serum cancer antigen 19-9, imaging studies such as MR, CT and endoscopic cholangiography with brushing or cytology (14). Of the imaging findings, malignant appearing mass compatible with cholangiocarcinoma is the most direct finding, although masses are uncommon in the early stage of cholangiocarcinoma. Progressive ductal dilatation seen in follow-up studies, marked ductal dilatation, severe ductal narrowing, mural thickening, and intraductal polypoid lesions in PSC are also highly suggestive of cholangiocarcinoma (916).
Medical therapy such as the use of ursodeoxycholic acid, corticosteroids or other immunosuppressive agents have shown only limited success in patients with PSC (22). In patients with advanced liver disease, liver transplantation is recommended as a curative treatment option (14). Recurrence of PSC after liver transplantation occurs in 20 to 25% of patients 5 to 10 years after the liver transplantation (23).
Immunoglobulin G4-SC is bile duct involvement of IgG4-related systemic disease (IgG4-RD) (24). After the pancreas, bile ducts are the second most common organ, involved with IgG4-RD. Patients with IgG4-SC are predominantly males in their 60s (mean age, 63 years) (25). Patients with IgG4-SC commonly present with obstructive jaundice. Like other IgG4-related diseases, the serum IgG4 is frequently elevated.
Immunoglobulin G4-SC shares the immunohistopathologic features of other IgG4-RD, including autoimmune pancreatitis, which is characterized by lymphoplasmacytic infiltration with abundant IgG4-positive plasma cells, storiform interstitial fibrosis, and obliterative phlebitis (Fig. 4) (26).
Abdominal US has limited value for diagnosing IgG4-SC, although it may show thickening of the bile duct and gallbladder. In cases of IgG4-SC, cross-sectional imaging, such as CT or MR imaging, demonstrates long-segmental, symmetrical, circumferential wall thickening and delayed contrast enhancement of the involved bile ducts (Fig. 5) (27). In approximately half of these patients, the involved bile duct lumens are narrowed, although visible (28). The most commonly involved location is the intrapancreatic segment of the common bile duct (CBD) followed by the hilar bile ducts in IgG4-SC (Fig. 5) (29). Multifocal involvement of both intrahepatic and extrahepatic bile ducts can occur, similar to PSC, while isolated stricture of the distal CBD can also be observed in IgG4-SC. The cholangiographic findings of IgG4-SC include long and continuous stricture of the bile ducts with prestenotic dilatation (Fig. 5) (30). Lymphadenopathy is not uncommon in patients with IgG4-SC (24). In particular, extrabiliary findings associated with IgG4-RD involvement, including the pancreas, kidney or retroperitoneal fibrosis, strongly suggest IgG4-SC (27).
Differentiation of IgG4-SC from other types of sclerosing cholangitis, especially from PSC, is clinically important as IgG4-SC shows a dramatic response to steroid therapy (Table 1) (31). Patients with PSC are generally younger and less symptomatic than those with IgG4-related disease. Multifocal intrahepatic duct involvement with short segmental strictures as well as a beaded, pruned-tree, and diverticulum-like appearance are imaging features suggestive of PSC. As IgG4-SC sometimes presents with prominent wall thickening in the bile duct, cholangiocarcinoma can be included as a differential diagnosis. Imaging features more suggestive of cholangiocarcinoma than of IgG4-SC include solitary lesion with irregular margins, eccentric wall thickening, invisible bile duct lumen in the involved segment, more prominent wall thickening (> 3 mm) and contrast enhancement, and an abrupt transition between the normal and involved bile duct (32). In patients with an inconclusive diagnosis, a steroid trial could be considered for the diagnosis of IgG4-SC in selected patients (33).
Recurrent pyogenic cholangitis is a progressive biliary disease characterized by recurrent episodes of cholangitis and intrahepatic pigmented stones (34). RPC is prevalent in Asian countries (35) and equally affects patients of both sexes (3637). Patients with RPC are commonly in the sixth and seventh decades of life and of rural and low socioeconomic status (3637). These patients typically present with abdominal pain, fever, and jaundice (the Charcot triad) and often have a history of recurrent episodes. The laboratory tests in RPC may demonstrate leukocytosis and elevated serum bilirubin.
Although the exact pathogenesis of RPC is unknown, strong associations with parasitic infestation such as Clonorchis sinensis or Ascaris lumbriocoides, portal bacteremia, and malnutrition are suggested (3638). Chronic recurrent infection of the bile ducts is thought to induce biliary stricture, bile stasis, and pigmented biliary stones. Approximately 30 to 80% of the stones in RPC are calcium bilirubinate stones commonly found in the intrahepatic bile duct (39). Histologically, bile ducts and periductal tissue show fibrous mural thickening, as well as acute and chronic inflammatory changes in RPC (38). The hepatic segments containing stones demonstrate parenchymal atrophy and scarring (38).
The diagnosis of RPC is based on the demographic background, as well as the clinical and imaging features. Abdominal US in RPC shows bile duct dilatation with increased periportal echogenicity. The intrahepatic bile duct stones can show various degrees of echogenicity and posterior shadowing (40). CT in RPC demonstrates disproportional dilatation of central intrahepatic ducts and extrahepatic bile ducts with nondilated or minimally dilated peripheral ducts, leading to decreased arborization and abrupt tapering of bile ducts (Fig. 6). Up to 90% of hepatolithiasis show hyperattenuation on unenhanced CT images, as compared to normal liver parenchyma (Fig. 6) (3641). Parenchymal atrophy occurs most frequently in the left lateral segment and the right posterior segment, while hypertrophy occurs in the caudate lobe and left medial segment, thus producing a round liver appearance in RPC. Heterogeneous parenchymal enhancement and periductal enhancement are often seen during acute exacerbations (42). For evaluation of patients with RPC, MR cholangiography is preferred to endoscopic cholangiography, since it can evaluate the bile ducts proximal to an obstruction and has no risk for biliary sepsis (40). However, endoscopic cholangiography has advantages for allowing therapeutic intervention. Cholangiography in RPC demonstrates dilatation of the central bile ducts and rapid tapering in the peripheral bile ducts, known as the arrowhead appearance (Fig. 6) (43). Strictures usually occur in the intrahepatic bile ducts (43). MR is more sensitive than CT for detecting radiolucent stones, which can appear hypointense on T2-weighted images and isointense to hyperintense on T1-weighted images (44). On the other hand, pneumobilia, which is commonly seen in RPC, can often mimic intrahepatic stones on MR or US, while it is more easily diagnosed with CT. In cases of Clonorchis sinensis infestation, cholangiography shows diffuse uniform dilatation of the peripheral intrahepatic bile ducts without a focal obstructive lesion. Flukes are occasionally visualized in the dilated bile ducts as filling defects (Fig. 7) (45).
The complications of RPC include biloma, hepatic abscess, inflammatory pseudotumor, thrombophlebitis of portal or hepatic veins, and cholangiocarcinoma (38). Hepatic abscesses can develop in up to 20% of these patients (43). The most threatening complication is cholangiocarcinoma, which is detected in 2 to 6% of patients with RPC (46). Cholangiocarcinoma most commonly occurs in atrophic hepatic segments or segments harboring many stones (Fig. 8) (35). Recurrent bacterial infection, bile stasis, and chronic irritation by stones are suggested as carcinogenic factors (47). Accurate diagnosis of cholangiocarcinoma in patients with RPC can be more difficult than in the general population, because biliary and parenchymal changes in underlying RPC can hinder the early detection of cholangiocarcinoma.
Recurrent pyogenic cholangitis sometimes mimics the imaging findings of PSC, which can also be accompanied by multifocal biliary strictures and hepatolithiasis. However, the involvement of both intrahepatic and extrahepatic bile ducts, a beaded appearance, and biliary diverticulum suggest PSC rather than RPC (Table 1) (38). Intraductal papillary neoplasm of the bile ducts can sometimes resemble RPC, as both present as nodular lesions in a dilated bile duct. The presence of contrast enhancement is suggestive of intraductal papillary neoplasm (38).
The treatment goals in RPC are complete removal of bile duct stone and bile stasis for the prevention of acute episodes of cholangitis and further stone formation (48). Therapeutic options include antibiotics for acute cholangitis, endoscopic procedures such as stricture dilatation or stone removal, biliary drainage, and surgical procedures such as biliary bypass, segmental hepatic resection or liver transplantation (40). A multidisciplinary approach is useful for proper management in patients with RPC. When a malignancy is suspected, biopsy or fine needle aspiration should be considered.
Ischemic cholangitis is defined as ischemia-induced bile duct injuries due to various causes. The bile ducts are vulnerable to ischemic injuries as the blood supply to the bile ducts depends completely on the arterial supply in contrast to the hepatic parenchyma, which has a dual blood supply from the hepatic artery and the portal vein (49). Among the various conditions that compromise the arterial supply and can cause ischemic cholangitis, iatrogenic causes including liver transplantation, hepatic arterial infusion of chemotherapeutic agents, and vessel injury during biliary or pancreatic surgery constitute the most common etiology (15051). Ischemic cholangitis can exist in patients with hereditary hemorrhagic telangiectasia or polyarteritis nodosa (151).
Pathological features of ischemic cholangitis vary depending on the degree and extent of hepatic arterial insufficiency and the stages of ischemic cholangitis (51). In the acute stage of ischemic cholangitis, desquamated ischemic biliary epithelium with other bile components forms biliary casts. Dilatation of the bile ducts is commonly seen at this stage, probably due to obstruction by the casts and a reaction to ischemic injury (51). When ischemic insult is more severe, necrosis involving the full thickness of the bile ducts occurs and spilled bile in the hepatic parenchyma creates biloma (51). As the disease progresses, focal or diffuse fibrous strictures may develop at a later stage of ischemic cholangitis (51).
Diagnosis can be made according to the imaging features compatible with ischemic injury and evidence of a compromised arterial supply (51). The clinical and imaging features are closely related to the stage of the ischemic cholangitis. At the acute stage of ischemic cholangitis, the patients usually present with fever, abdominal pain, jaundice, and biliary sepsis. At this stage, the radiologic findings of biliary casts include intraductal filling defects in the dilated bile duct showing high signal intensity on nonenhanced T1-weighed MR images (Fig. 9) (52). At the acute stage of ischemic cholangitis, biliary casts appear similar to intraductal stones. However, they usually differ in their shapes, as biliary casts appear linear or have a branching pattern, whereas stones are usually oval or round (52). In severe ischemic injury, bile duct necrosis presents as tubular low density or intensity structures along the portal tracts on enhanced CT or MR images (Fig. 10) (51). Cholangiography in ischemic cholangitis shows dilated bile ducts with irregular margins and intraluminal filling defects (51). Bilomas can appear as an intrahepatic fluid collection usually located in the vicinity of the injured bile ducts (Figs. 9, 10) (53). At the chronic stage of ischemic cholangitis, the clinical symptoms and laboratory profile include progressive or fluctuating cholestasis. As the disease progresses, focal or diffuse strictures may develop at a later stage. Cholangiographic findings of ischemic cholangitis at this stage include multifocal biliary strictures and dilatation, both of which can mimic the findings of PSC (Fig. 10) (51). The clinical context and predominant location of ischemic strictures, i.e., the middle third of the CBD and the hilar bile duct, are suggestive of ischemic cholangitis rather than PSC (51).
Appropriate treatment option in ischemic cholangitis should be based on the individual patient's clinical setting. In the transplanted patients with large artery occlusion, restoration of arterial flow can be attempted using thrombolytic agents, balloon angioplasty or stenting and surgical revision. On the other hand, in patients with small artery ischemic injuries, management of complications caused by bile duct injury may be more important as there is no specific curative method. Endoscopic procedures allow removal of biliary casts, and percutaneous drainage is useful for biloma drainage and decompressing dilated bile ducts in ischemic cholangitis. Biliary bypass surgery can be performed for bile duct reconstruction in ischemic cholangitis (51).
Acquired immunodeficiency syndrome-related cholangitis is a form of secondary sclerosing cholangitis occurring in patients with human immunodeficiency virus (HIV) infection. AIDS-related cholangitis typically affects advanced HIV-infected patients with markedly decreased immune function (CD4 count < 100/mm3) (5455). The suggested mechanisms of AIDS-related cholangitis include randomly occurring infections involving the bile ducts, ischemia, autonomic nerve injury, and direct invasion of bile duct epithelium by the HIV itself (5657). The most common pathogens in AIDS-related cholangitis are cytomegalovirus, Cryptosporidium parvum, Mycobacterium avium complex, and herpes simplex virus, although no definite pathogen is identified in almost 50% of these patients (34). The typical clinical manifestations in AIDS-related cholangitis include right upper abdominal pain, as well as elevated alkaline phosphatase and transaminase levels. Associated papillary stenosis may result in more intense abdominal pain (58).
On CT and MR imaging, enhanced wall thickening of the extrahepatic bile duct and the intrahepatic bile ducts can be seen in AIDS-related cholangitis (Fig. 11) (5960). Cholangiographic findings in AIDS-related cholangitis include multifocal strictures and dilatation of intrahepatic and extrahepatic bile ducts resembling those seen in patients with PSC (Fig. 11) (3459). In AIDS-related cholangitis, endoscopic cholangiography features of papillary stricture include tapered narrowing in the terminal portion of dilated CBD and marked and delayed contrast retention (Fig. 11) (5961). The patient's clinical history, as well as some imaging features including papillary stenosis and a long extrahepatic bile duct stricture, can also help to differentiate AIDS-related cholangitis from PSC (5859).
Although antimicrobial therapy could be used for opportunistic infections in AIDS-related cholangitis, it is reported to be mostly ineffective (62). Sphincterotomy is the primary therapy for patients with papillary stenosis (58). For biliary stricture in AIDS-related cholangitis, endoscopic balloon dilatation and stent placement may be performed (63).
Eosinophilic cholangitis is defined as eosinophilic infiltration of the biliary system. Although there have been anecdotal case series in the English literature (646566), patients with eosinophilic cholangitis generally present with jaundice, which is often associated with eosinophilic gastroenteritis (64). Peripheral eosinophilia is the most helpful laboratory finding, even though it is reported in only approximately half of the patients with eosinophilic cholangitis (64). Histopathologically, eosinophilic cholangitis is characterized by dense transmural eosinophilic infiltration associated with other inflammatory cell infiltration in the bile ducts (65). A thickened fibromuscular layer and slight fibrosis in the subserosal layer are also present with eosinophilic cholangitis (65).
Imaging findings in eosinophilic cholangitis mentioned in previous reports include: wall thickening of the CBD, cystic duct, and gallbladder; diffuse bile duct stricture from the hepatic hilum to the intrahepatic duct; focal stricture of the common hepatic duct at the cystic duct insertion level; and mild smooth narrowing of the proximal common duct (Fig. 12) (116646567). Although a preoperative diagnosis of eosinophilic cholangitis is challenging due to its nonspecific imaging features, it should be considered when bile duct wall thickening is present with involvement of the cystic duct, the gallbladder, and peripheral eosinophilia. Patients with eosinophilic cholangitis show a favorable response to corticosteroids and often show a self-limited disease course without treatment (16666869).
Before arriving at the differential diagnosis of sclerosing cholangitis, it is critical to exclude malignant bile duct strictures, especially when a single segmental bile duct wall thickening and/or stricture is detected. The following image features favor malignant biliary strictures rather than sclerosing cholangitis: a narrowed segment with hyperenhancement relative to the liver seen during the portal-venous phase; long length involved (> 12 mm); prominent bile duct thickening (> 3 mm); an indistinct outer margin; luminal irregularity; and asymmetry (70). After excluding malignant biliary strictures, the next step is to distinguish between PSC and secondary sclerosing cholangitis. Figure 13 summarizes the characteristic imaging features of sclerosing cholangitis according to the underlying etiologies. It is often challenging to differentiate the underlying etiologies of sclerosing cholangitis based solely on the morphological features. Therefore, the demographics, laboratory profiles, and patient's past medical history should be considered together with the imaging features. A systematic approach (Fig. 14) combined with the appropriate clinical settings and imaging findings, can be helpful for differentiating the various causes of sclerosing cholangitis. Pathologic confirmation, such as biopsy, is necessary in patients with an inconclusive diagnosis. In cases suspected of IgG4-SC, steroid therapy can be attempted for diagnostic and therapeutic purposes in a selected patient group.
There is a wide spectrum of causes of sclerosing cholangitis, including PSC and secondary sclerosing cholangitis caused by various conditions including IgG4-SC, RPC, ischemic cholangitis, AIDS-related cholangitis, and eosinophilic cholangitis. Several characteristic imaging features are suggestive of the underlying causes of sclerosing cholangitis, however, these imaging features substantially overlap. A systematic approach combined with appropriate clinical settings and imaging findings, may help to differentiate various causes of sclerosing cholangitis and ultimately to guide the appropriate patient management.
Figures and Tables
 | Fig. 1Pathology of primary sclerosing cholangitis.Photomicrograph of liver biopsy specimen (original magnification, × 400; hematoxylin and eosin stain) reveals fibrous portal widening with concentric onion-skin fibrosis around interlobular bile duct and moderate degree of mixed inflammatory cell infiltration (arrows).
|
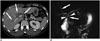 | Fig. 2Primary sclerosing cholangitis in 36-year-old male.
A. Contrast-enhanced axial CT image demonstrates mild multifocal wall thickening in intrahepatic and extrahepatic bile ducts (arrows). B. MR cholangiography shows multiple and short segmental strictures in intra- and extrahepatic bile ducts (arrows), as well as diverticular outpouching (arrowheads).
|
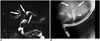 | Fig. 3Chronologic change of PSC in 36-year-old male.
A. MR cholangiography image depicts multifocal alternating strictures and dilatation of intrahepatic bile ducts (arrows). B. Follow-up endoscopic cholangiography obtained 5 years after MR cholangiography (A) shows obliterated peripheral bile ducts, resulting in "pruned tree" appearance (arrows). Extrahepatic bile duct is dilated (arrowhead). MR = magnetic resonance, PSC = primary sclerosing cholangitis
|
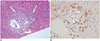 | Fig. 4Pathology of IgG4-related SC.
A. Photomicrograph of liver biopsy specimen (original magnification, × 400; hematoxylin and eosin stain) shows fibrous portal widening with concentric fibrosis and moderate degree of mixed inflammatory cells infiltration (arrows) around interlobular bile duct (arrowhead) (curved arrow, arteriole; asterisk, portal venule). B. There are abundant IgG4-positive plasma cells around bile ducts (arrowheads) (original magnification, × 400; IgG4 staining). IgG4 = immunoglobulin G4, SC = sclerosing cholangitis
|
 | Fig. 5IgG4-related SC in 77-year-old male.
A. Contrast-enhanced axial CT image shows mildly enhanced wall thickening of common hepatic duct (arrow) with mild dilatation of intrahepatic bile ducts (arrowheads). B. MR cholangiography shows focal stricture of intrapancreatic bile duct (arrow), as well as multifocal stricture in right intrahepatic bile ducts (arrowheads). IgG4 = immunoglobulin G4, MR = magnetic resonance, SC = sclerosing cholangitis
|
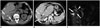 | Fig. 6Recurrent pyogenic cholangitis in 63-year-old male.
A. Nonenhanced CT image shows radiopaque stones (arrows) in dilated bile ducts in right posterior segment of liver. B. Contrast-enhanced CT image shows dilated central bile ducts (arrows) and rounded liver appearance with marked hypertrophy of caudate lobe (arrowhead). C. MR cholangiography image demonstrates bile duct stones as multiple filling defects (arrows) with bile duct stricture (arrowhead). CT = computed tomography, MR = magnetic resonance
|
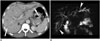 | Fig. 7Recurrent pyogenic cholangitis with Clonorchis sinensis infestation in 62-year-old male.
A. Contrast-enhanced CT image shows diffuse dilatation of intrahepatic bile ducts, especially in peripheral portion of bile ducts (arrows). B. MR cholangiography shows marked peripheral intrahepatic bile duct dilatation without central bile duct dilatation. Multiple filling defects (arrowheads) noted within dilated bile ducts suggest presence of Clonorchis sinensis worms. CT = computed tomography, MR = magnetic resonance
|
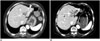 | Fig. 8Cholangiocarcinoma arising from recurrent pyogenic cholangitis in 82-year-old female.
A. Dilated left IHD (arrowheads) containing IHD stones is noted on contrast-enhanced CT image. B. Patient underwent follow-up CT scan after 4 years. Axial contrast-enhanced CT scan shows newly developed, soft-tissue lesion (arrow) in left lobe of liver causing bile duct dilatation, as well as narrowing of left portal vein. Lesion was confirmed as cholangiocarcinoma after surgery. CT = computed tomography, IHD = intrahepatic bile duct
|
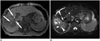 | Fig. 9Ischemic cholangitis after transcatheter arterial chemoembolization for hepatocellular carcinoma in 74-year-old male.
A. Nonenhanced T1-weighted axial MR image detects multiple, hyperintense biliary casts in right intrahepatic bile duct (arrows). B. T2-weighted axial MR image depicts multiple bilomas (arrowheads). MR = magnetic resonance
|
 | Fig. 10Ischemic cholangitis after hepatic arterial embolization for postoperative pseudoaneurysm in 50-year-old female.
A. At acute stage, contrast-enhanced axial CT image shows bile duct necrosis (arrows) and biloma (arrowhead). B, C. Contrast-enhanced axial CT scan (B) and percutaneous cholangiography (C) obtained 3 months following embolization, demonstrate multifocal bile duct stricture (arrows) and dilatation (arrowheads) that are typical features of chronic ischemic cholangitis. CT = computed tomography
|
 | Fig. 11AIDS-related cholangitis with cytomegalovirus (CMV) infection in 43-year-old male.
A, B. Contrast-enhanced axial CT images show multifocal stricture and wall thickening in intrahepatic bile ducts (arrows). Papillary stenosis with enhancement (arrowhead) is also seen. C. On endoscopic cholangiography, diffuse stricture is noted in intrahepatic bile ducts (arrow). Common duct also shows dilatation and subtle mucosal irregularity (arrowhead). Endoscopic biopsy confirmed CMV infection. AIDS = acquired immunodeficiency syndrome, CT = computed tomography
|
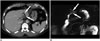 | Fig. 12Eosionophilic cholangitis in 43-year-old male with hypereosinophilia.
A. Contrast-enhanced axial CT image demonstrates ill-defined soft-tissue lesions along periportal space (arrows). B. MR cholangiography shows focal smooth narrowing in left hepatic duct (arrows). Lesion was confirmed as eosinophilic infiltration after liver biopsy. CT = computed tomography, MR = magnetic resonance
|
 | Fig. 13Summary of imaging features of sclerosing cholangitis.Red lines along bile ducts indicate sites of frequent involvement according to etiologies of sclerosing cholangitis. Primary sclerosing cholangitis (PSC). Both intra- and extrahepatic bile ducts are usually involved in PSC. Beaded and pruned appearance of bile ducts, and diverticulum-like outpouching are characteristic imaging features of PSC. Immunoglobulin G4-related sclerosing cholangitis (IgG4-SC). Most commonly involved location is intrapancreatic bile duct followed by hilar bile ducts. Circumferential and delayed enhanced wall thickening with visible lumen is suggestive of IgG4-related SC. Findings of autoimmune pancreatitis can frequently be accompanied with IgG4-related SC. Recurrent pyogenic cholangitis (RPC). Most frequently involved segments are left lateral segment and right posterior segment of liver. RPC is characterized by intrahepatic bile duct stones, central bile duct dilatation, and decreased arborization of peripheral ducts. Ischemic cholangitis. Most vulnerable location is middle third of common bile duct. At acute stage, biliary casts and bilomas are common. As disease progresses to chronic stage, focal or diffuse bile duct stricture may develop. AIDS-related cholangitis. Long segmental stricture of extrahepatic bile duct and papillary stenosis are key imaging findings. Eosinophilic cholangitis. Wall thickening in proximal common bile duct and cystic duct is commonly seen. AIDS = acquired immunodeficiency syndrome
|
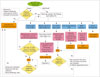 | Fig. 14Systemic approach of sclerosing cholangitis.This diagram shows approach to differential diagnosis of sclerosing cholangitis based on clinical setting, laboratory findings, as well as typical imaging features. AIDS = acquired immunodeficiency syndrome, EHD = extrahepatic bile duct, Hx = history, IBD = inflammatory bowel disease, IgG4-SC = immunoglobulin G4-related sclerosing cholangitis, IHD = intrahepatic bile duct, PSC = primary sclerosing cholangitis, RPC = recurrent pyogenic cholangitis, TACE = transarterial chemoembolization
|
Table 1
Comparison of Primary Sclerosing Cholangitis, IgG4-Related Sclerosing Cholangitis, and Recurrent Pyogenic Cholangitis

References
1. Azizi L, Raynal M, Cazejust J, Ruiz A, Menu Y, Arrivé L. MR Imaging of sclerosing cholangitis. Clin Res Hepatol Gastroenterol. 2012; 36:130–138.
2. Maggs JR, Chapman RW. An update on primary sclerosing cholangitis. Curr Opin Gastroenterol. 2008; 24:377–383.
3. Tischendorf JJ, Hecker H, Krüger M, Manns MP, Meier PN. Characterization, outcome, and prognosis in 273 patients with primary sclerosing cholangitis: a single center study. Am J Gastroenterol. 2007; 102:107–114.
4. Kingham JG, Kochar N, Gravenor MB. Incidence, clinical patterns, and outcomes of primary sclerosing cholangitis in South Wales, United Kingdom. Gastroenterology. 2004; 126:1929–1930.
5. Bambha K, Kim WR, Talwalkar J, Torgerson H, Benson JT, Therneau TM, et al. Incidence, clinical spectrum, and outcomes of primary sclerosing cholangitis in a United States community. Gastroenterology. 2003; 125:1364–1369.
6. Escorsell A, Parés A, Rodés J, Solís-Herruzo JA, Miras M, de la Morena E. Spanish Association for the Study of the Liver. Epidemiology of primary sclerosing cholangitis in Spain. J Hepatol. 1994; 21:787–791.
7. Ang TL, Fock KM, Ng TM, Teo EK, Chua TS, Tan JY. Clinical profile of primary sclerosing cholangitis in Singapore. J Gastroenterol Hepatol. 2002; 17:908–913.
8. Wiesner RH, LaRusso NF. Clinicopathologic features of the syndrome of primary sclerosing cholangitis. Gastroenterology. 1980; 79:200–206.
9. Elsayes KM, Oliveira EP, Narra VR, Abou El Abbass HA, Ahmed MI, Tongdee R, et al. MR and MRCP in the evaluation of primary sclerosing cholangitis: current applications and imaging findings. J Comput Assist Tomogr. 2006; 30:398–404.
10. Saarinen S, Olerup O, Broomé U. Increased frequency of autoimmune diseases in patients with primary sclerosing cholangitis. Am J Gastroenterol. 2000; 95:3195–3199.
11. Chapman RW, Jewell DP. Primary sclerosing cholangitis--an immunologically mediated disease? West J Med. 1985; 143:193–195.
12. Karlsen TH, Franke A, Melum E, Kaser A, Hov JR, Balschun T, et al. Genome-wide association analysis in primary sclerosing cholangitis. Gastroenterology. 2010; 138:1102–1111.
13. Hirschfield GM, Karlsen TH, Lindor KD, Adams DH. Primary sclerosing cholangitis. Lancet. 2013; 382:1587–1599.
14. Chapman R, Fevery J, Kalloo A, Nagorney DM, Boberg KM, Shneider B, et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology. 2010; 51:660–678.
15. European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol. 2009; 51:237–267.
16. Vitellas KM, Keogan MT, Freed KS, Enns RA, Spritzer CE, Baillie JM, et al. Radiologic manifestations of sclerosing cholangitis with emphasis on MR cholangiopancreatography. Radiographics. 2000; 20:959–975. quiz 1108-1109, 1112
17. Gulliver DJ, Baker ME, Putnam W, Baillie J, Rice R, Cotton PB. Bile duct diverticula and webs: nonspecific cholangiographic features of primary sclerosing cholangitis. AJR Am J Roentgenol. 1991; 157:281–285.
18. Fulcher AS, Turner MA, Franklin KJ, Shiffman ML, Sterling RK, Luketic VA, et al. Primary sclerosing cholangitis: evaluation with MR cholangiography-a case-control study. Radiology. 2000; 215:71–80.
19. Ament AE, Haaga JR, Wiedenmann SD, Barkmeier JD, Morrison SC. Primary sclerosing cholangitis: CT findings. J Comput Assist Tomogr. 1983; 7:795–800.
20. Revelon G, Rashid A, Kawamoto S, Bluemke DA. Primary sclerosing cholangitis: MR imaging findings with pathologic correlation. AJR Am J Roentgenol. 1999; 173:1037–1042.
21. Claessen MM, Vleggaar FP, Tytgat KM, Siersema PD, van Buuren HR. High lifetime risk of cancer in primary sclerosing cholangitis. J Hepatol. 2009; 50:158–164.
22. Cullen SN, Chapman RW. The medical management of primary sclerosing cholangitis. Semin Liver Dis. 2006; 26:52–61.
23. Campsen J, Zimmerman MA, Trotter JF, Wachs M, Bak T, Steinberg T, et al. Clinically recurrent primary sclerosing cholangitis following liver transplantation: a time course. Liver Transpl. 2008; 14:181–185.
24. Stone JH. IgG4-related disease: nomenclature, clinical features, and treatment. Semin Diagn Pathol. 2012; 29:177–190.
25. Nishino T, Oyama H, Hashimoto E, Toki F, Oi I, Kobayashi M, et al. Clinicopathological differentiation between sclerosing cholangitis with autoimmune pancreatitis and primary sclerosing cholangitis. J Gastroenterol. 2007; 42:550–559.
26. Zen Y, Nakanuma Y, Portmann B. Immunoglobulin G4-related sclerosing cholangitis: pathologic features and histologic mimics. Semin Diagn Pathol. 2012; 29:205–211.
27. Vlachou PA, Khalili K, Jang HJ, Fischer S, Hirschfield GM, Kim TK. IgG4-related sclerosing disease: autoimmune pancreatitis and extrapancreatic manifestations. Radiographics. 2011; 31:1379–1402.
28. Itoh S, Nagasaka T, Suzuki K, Satake H, Ota T, Naganawa S. Lymphoplasmacytic sclerosing cholangitis: assessment of clinical, CT, and pathological findings. Clin Radiol. 2009; 64:1104–1114.
29. Kawamoto S, Siegelman SS, Hruban RH, Fishman EK. Lymphoplasmacytic sclerosing pancreatitis (autoimmune pancreatitis): evaluation with multidetector CT. Radiographics. 2008; 28:157–170.
30. Nakazawa T, Ohara H, Sano H, Aoki S, Kobayashi S, Okamoto T, et al. Cholangiography can discriminate sclerosing cholangitis with autoimmune pancreatitis from primary sclerosing cholangitis. Gastrointest Endosc. 2004; 60:937–944.
31. Nishino T, Toki F, Oyama H, Oi I, Kobayashi M, Takasaki K, et al. Biliary tract involvement in autoimmune pancreatitis. Pancreas. 2005; 30:76–82.
32. Kim JH, Byun JH, Lee SJ, Park SH, Kim HJ, Lee SS, et al. Differential diagnosis of sclerosing cholangitis with autoimmune pancreatitis and periductal infiltrating cancer in the common bile duct at dynamic CT, endoscopic retrograde cholangiography and MR cholangiography. Eur Radiol. 2012; 22:2502–2513.
33. Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, et al. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011; 40:352–358.
34. Catalano OA, Sahani DV, Forcione DG, Czermak B, Liu CH, Soricelli A, et al. Biliary infections: spectrum of imaging findings and management. Radiographics. 2009; 29:2059–2080.
35. Al-Sukhni W, Gallinger S, Pratzer A, Wei A, Ho CS, Kortan P, et al. Recurrent pyogenic cholangitis with hepatolithiasis--the role of surgical therapy in North America. J Gastrointest Surg. 2008; 12:496–503.
36. Okuno WT, Whitman GJ, Chew FS. Recurrent pyogenic cholangiohepatitis. AJR Am J Roentgenol. 1996; 167:484.
37. Kim MH, Sekijima J, Lee SP. Primary intrahepatic stones. Am J Gastroenterol. 1995; 90:540–548.
38. Tsui WM, Chan YK, Wong CT, Lo YF, Yeung YW, Lee YW. Hepatolithiasis and the syndrome of recurrent pyogenic cholangitis: clinical, radiologic, and pathologic features. Semin Liver Dis. 2011; 31:33–48.
39. Leung JW, Yu AS. Hepatolithiasis and biliary parasites. Baillieres Clin Gastroenterol. 1997; 11:681–706.
40. Heffernan EJ, Geoghegan T, Munk PL, Ho SG, Harris AC. Recurrent pyogenic cholangitis: from imaging to intervention. AJR Am J Roentgenol. 2009; 192:W28–W35.
41. Afagh A, Pancu D. Radiologic findings in recurrent pyogenic cholangitis. J Emerg Med. 2004; 26:343–346.
42. Chan FL, Man SW, Leong LL, Fan ST. Evaluation of recurrent pyogenic cholangitis with CT: analysis of 50 patients. Radiology. 1989; 170(1 Pt 1):165–169.
43. Kim MJ, Cha SW, Mitchell DG, Chung JJ, Park S, Chung JB. MR imaging findings in recurrent pyogenic cholangitis. AJR Am J Roentgenol. 1999; 173:1545–1549.
44. Yeh BM, Liu PS, Soto JA, Corvera CA, Hussain HK. MR imaging and CT of the biliary tract. Radiographics. 2009; 29:1669–1688.
45. Lim JH, Mairiang E, Ahn GH. Biliary parasitic diseases including clonorchiasis, opisthorchiasis and fascioliasis. Abdom Imaging. 2008; 33:157–165.
46. Menias CO, Surabhi VR, Prasad SR, Wang HL, Narra VR, Chintapalli KN. Mimics of cholangiocarcinoma: spectrum of disease. Radiographics. 2008; 28:1115–1129.
47. Kim JH, Kim TK, Eun HW, Byun JY, Lee MG, Ha HK, et al. CT findings of cholangiocarcinoma associated with recurrent pyogenic cholangitis. AJR Am J Roentgenol. 2006; 187:1571–1577.
48. Park MS, Yu JS, Kim KW, Kim MJ, Chung JP, Yoon SW, et al. Recurrent pyogenic cholangitis: comparison between MR cholangiography and direct cholangiography. Radiology. 2001; 220:677–682.
49. Northover JM, Terblanche J. A new look at the arterial supply of the bile duct in man and its surgical implications. Br J Surg. 1979; 66:379–384.
50. Deltenre P, Valla DC. Ischemic cholangiopathy. J Hepatol. 2006; 44:806–817.
51. Deltenre P, Valla DC. Ischemic cholangiopathy. Semin Liver Dis. 2008; 28:235–246.
52. Kinner S, Umutlu L, Dechêne A, Ladd SC, Barkhausen J, Gerken G, et al. Biliary complications after liver transplantation: addition of T1-weighted images to MR cholangiopancreatography facilitates detection of cast in biliary cast syndrome. Radiology. 2012; 263:429–436.
53. Valente JF, Alonso MH, Weber FL, Hanto DW. Late hepatic artery thrombosis in liver allograft recipients is associated with intrahepatic biliary necrosis. Transplantation. 1996; 61:61–65.
54. Keaveny AP, Karasik MS. Hepatobiliary and pancreatic infections in AIDS: Part one. AIDS Patient Care STDS. 1998; 12:347–357.
55. Abdalian R, Heathcote EJ. Sclerosing cholangitis: a focus on secondary causes. Hepatology. 2006; 44:1063–1074.
56. Keaveny AP, Karasik MS. Hepatobiliary and pancreatic infections in AIDS: Part II. AIDS Patient Care STDS. 1998; 12:451–456.
57. Mahajani RV, Uzer MF. Cholangiopathy in HIV-infected patients. Clin Liver Dis. 1999; 3:669–684. x
58. Cello JP, Chan MF. Long-term follow-up of endoscopic retrograde cholangiopancreatography sphincterotomy for patients with acquired immune deficiency syndrome papillary stenosis. Am J Med. 1995; 99:600–603.
59. Bilgin M, Balci NC, Erdogan A, Momtahen AJ, Alkaade S, Rau WS. Hepatobiliary and pancreatic MRI and MRCP findings in patients with HIV infection. AJR Am J Roentgenol. 2008; 191:228–232.
60. Bader TR, Braga L, Beavers KL, Semelka RC. MR imaging findings of infectious cholangitis. Magn Reson Imaging. 2001; 19:781–788.
61. Wilcox CM, Mönkemüller KE. Hepatobiliary diseases in patients with AIDS: focus on AIDS cholangiopathy and gallbladder disease. Dig Dis. 1998; 16:205–213.
62. Forbes A, Blanshard C, Gazzard B. Natural history of AIDS related sclerosing cholangitis: a study of 20 cases. Gut. 1993; 34:116–121.
63. Cordero E, López-Cortés LF, Belda O, Villanueva JL, Rodríguez-Hernández MJ, Pachn J. Acquired immunodeficiency syndrome-related cryptosporidial cholangitis: resolution with endobiliary prosthesis insertion. Gastrointest Endosc. 2001; 53:534–535.
64. Vauthey JN, Loyer E, Chokshi P, Lahoti S. Case 57: eosinophilic cholangiopathy. Radiology. 2003; 227:107–112.
65. Miura F, Asano T, Amano H, Yoshida M, Toyota N, Wada K, et al. Resected case of eosinophilic cholangiopathy presenting with secondary sclerosing cholangitis. World J Gastroenterol. 2009; 15:1394–1397.
66. Butler TW, Feintuch TA, Caine WP Jr. Eosinophilic cholangitis, lymphadenopathy, and peripheral eosinophilia: a case report. Am J Gastroenterol. 1985; 80:572–574.
67. Nashed C, Sakpal SV, Shusharina V, Chamberlain RS. Eosinophilic cholangitis and cholangiopathy: a sheep in wolves clothing. HPB Surg. 2010; 2010:906496.
68. Rosengart TK, Rotterdam H, Ranson JH. Eosinophilic cholangitis: a self-limited cause of extrahepatic biliary obstruction. Am J Gastroenterol. 1990; 85:582–585.
69. Song HH, Byun JY, Jung SE, Choi KH, Shinn KS, Kim BK. Eosinophilic cholangitis: US, CT, and cholangiography findings. J Comput Assist Tomogr. 1997; 21:251–253.
70. Kim JY, Lee JM, Han JK, Kim SH, Lee JY, Choi JY, et al. Contrast-enhanced MRI combined with MR cholangiopancreatography for the evaluation of patients with biliary strictures: differentiation of malignant from benign bile duct strictures. J Magn Reson Imaging. 2007; 26:304–312.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download