Abstract
Objective
To evaluate the feasibility, safety, and clinical outcomes of plug-assisted retrograde transvenous obliteration (PARTO) to treat gastric variceal hemorrhage in patients with portal hypertension.
Materials and Methods
From May 2012 to June 2014, 19 patients (11 men and 8 women, median age; 61, with history of gastric variceal hemorrhage; 17, active bleeding; 2) who underwent PARTO using a vascular plug and a gelfoam pledget were retrospectively analyzed. Clinical and laboratory data were examined to evaluate primary (technical and clinical success, complications) and secondary (worsening of esophageal varix [EV], change in liver function) end points. Median follow-up duration was 11 months, from 6.5 to 18 months. The Wilcoxon signed-rank test was used to compare laboratory data before and after the procedure.
Results
Technical success (complete occlusion of the efferent shunt and complete filling of gastric varix [GV] with a gelfoam slurry) was achieved in 18 of 19 (94.7%) patients. The embolic materials could not reach the GV in 1 patient who had endoscopic glue injection before our procedure. The clinical success rate (no recurrence of gastric variceal bleeding) was the same because the technically failed patient showed recurrent bleeding later. Acute complications included fever (n = 2), fever and hypotension (n = 2; one diagnosed adrenal insufficiency), and transient microscopic hematuria (n = 3). Ten patients underwent follow-up endoscopy; all exhibited GV improvement, except 2 without endoscopic change. Five patients exhibited aggravated EV, and 2 of them had a bleeding event. Laboratory findings were significantly improved after PARTO.
Gastric variceal bleeding is a common (25%), yet severe complication of portal hypertension (1). It is one of the leading causes of death in patients with liver cirrhosis (23). Despite the advances in treatment over the last few decades, the mortality rate is still high with reported 5-year survival rate of 53.2% (4). The current guidelines recommend initial medical therapy followed by endoscopic glue injection for management of gastric varix (GV) (56). Radiologic intervention includes transjugular intrahepatic portosystemic shunt (TIPS) and balloon-occluded retrograde transvenous obliteration (BRTO). In Asian countries, especially in South Korea and Japan, BRTO is performed more frequently than TIPS to treat gastric variceal hemorrhage, because BRTO is less invasive and reportedly improves hepatic function of the patients (7). One possible devastating complication of conventional BRTO is balloon rupture and subsequent systemic circulation of sclerosant. Reported incidence of this complication is 8.7% (8). Relatively common complications include hemolysis and hemoglobinuria. Rare, but previously reported complications include acute renal failure, pulmonary edema, and anaphylaxis to ethanolamine oleate (91011). Dose limitation of sclerosing agents is another weak point of conventional BRTO. Finally, patients have to be placed on bed rest for at least 30 minutes to 3 hours (12) or 4 to 24 hours before the balloon catheters are retrieved. To the best of our knowledge, only 2 studies from 2 centers have been reported to evaluate the effectiveness of vascular plug and gelatin sponge-assisted retrograde transvenous obliteration (1314). Therefore, the purpose of our study was to evaluate the feasibility and safety of plug-assisted retrograde transvenous obliteration (PARTO) for treating gastric variceal hemorrhage in patients with portal hypertension and its clinical outcomes.
The Institutional Review Board at our hospital approved this retrospective study and waived the requirement of informed consent. We retrospectively reviewed the medical records of all 19 patients who underwent PARTO for gastric variceal hemorrhage between May 2012 and June 2014. All of them had liver cirrhosis and the history of remote or current gastric variceal bleeding on endoscopy. Only those with gastrorenal shunt (GRS) confirmed on pre-procedural CT scan were included. We excluded patients with high risk of esophageal variceal hemorrhage (nodular/tumorous form or previous history of bleeding) or intractable ascites. Total 19 patients (11 men and 8 women, median age; 61, with history of gastric variceal hemorrhage; 17, active bleeding; 2) who underwent PARTO using a vascular plug and a gelfoam pledget were included in the study population.
Table 1 summarized the patient characteristics of the study population. Seventeen patients with history of gastric variceal bleeding underwent elective procedures. Two patients initially presented to the emergency room with active upper gastrointestinal bleeding that resulted in hemodynamic instability. Their initial vital signs were as follows: blood pressure 95/70 mm Hg with pulse 114/minute for 1 patient, and blood pressure 96/48 mm Hg with pulse 111/minute for the other. After blood transfusion and terlipressin acetate (Glypressin, Ferring Korea, Seoul, Korea) administration, initial dose of 2 mg followed by additional 1 mg every 4 hours during 3 days, emergent endoscopic glue injection was performed, but we could not control the bleeding because of the large amount of hematomas and food materials. A Sengstaken-Blakemore tube was applied instead, and PARTO was performed in an urgent/emergent setting. Other 2 patients (10.5%) underwent endoscopic glue injection for GV 49 days or 4 days prior to our procedure, respectively. Only 1 patient with Child-Pugh classification B showed hepatic encephalopathy, West Haven Criteria grade 1–2. Six patients had small amount of ascites before the procedure.
All patients underwent contrast-enhanced computed tomography imaging to evaluate the presence, as well as the size and the morphology of GV and GRS. Vascular plug of approximately 30% larger diameter than the GRS waist measured on CT images was selected. All patients had initial endoscopic evaluations. The presence and severity of GV and esophageal varix (EV) were assessed according to the rule proposed by The Japan Society for Portal Hypertension (15). Thirteen patients had F3 GV, of which, 2 had active gastric variceal bleeding. Five patients had F2 GV, and 1 patient was assessed as F1 with positive red color sign. Nine out of 19 patients had both GV and EV at initial endoscopic evaluation.
All procedures were performed under conscious sedation using intravenous pethidine hydrochloride 25 mg (Pethidine, Je-il Pharmaceutical, Daegu, Korea), and local anesthesia at puncture site using lidocaine hydrochloride (Lidocaine, Je-il Pharmaceutical, Daegu, Korea). Right femoral vein was punctured under ultrasonography, and a 9 Fr TIPS sheath (Cook, Bloomington, IN, USA) was placed in the left renal vein or GRS. In cases where the catheter was not advanced to GRS due to vascular tortuosity, a 6–8 Fr guiding catheter (Brite Tip, Cordis, Miami Lakes, FL, USA) was used (n = 6, 31.6%). After negotiation, a 0.035-inch guidewire (Terumo, Tokyo, Japan) was advanced to GRS adjacent to GV. A 10–22 mm Amplatzer vascular plug type 2 (AVP; St. Jude Medical, Inc., St. Paul, MN, USA) with a size compatible with the measured GRS diameter was deployed at the narrowest part, while we kept the guidewire next to the vascular plug. Finally, a 4 Fr angled-tip catheter (Cobra; Terumo, Tokyo, Japan) was advanced to the GRS along the guidewire (Fig. 1). Contrast media was injected through the catheter 5 to 10 minutes after the plug deployment to confirm that the efferent shunt was completely blocked. In cases with minimal contrast leakage to the left renal vein, gradual gelfoam slurry embolization was attempted in turn until complete occlusion. For insufficient cases, supplementary embolization with 12 mm × 0.035-inch platinum coil (Nester, Cook) was done through the 4 Fr catheter to ensure complete obstruction. Subsequently, embolization was performed using gelfoam slurry, the mixture of 1 mm3 gelatin sponges with half of contrast media and half of saline. It was injected carefully under fluoroscopic guidance until the left gastric vein or the posterior gastric vein was visualized. In cases with collateral veins, such as pericardiacophrenic vein, inferior phrenic vein, or intercostal vein, the gelfoam mixture was injected slowly and contrast media was injected in between to ensure that the collateral veins were occluded. We considered coil embolization for a large collateral vein that was not spontaneously occluded with gelfoam embolization. Technical success was defined as the successful placement of the vascular plug and complete filling of the GV with gelfoam pledgets on angiography. Clinical success was defined as no recurrent gastric variceal hemorrhage until the end of study period. Procedure time was calculated using the fluoroscopy time.
All patients were subjected to contrast-enhanced CT scanning within 1 month after the procedure to primarily evaluate GV obliteration, and also procedure-related complication such as hematoma or systemic thrombus, and advent of ectopic varices or ascites. Medical records were reviewed for acute complication, recurrent bleeding, and changes in liver function test after the procedure. Endoscopic follow-up was performed for 10 patients according to the discretion of the referring physicians, and changes in GV and EV were assessed. Median interval from the procedure to endoscopy was 3 months, from 4 days to 1 year. Follow-up laboratory tests were done within a week (from 3 days to 1 week, median 4 days) after the procedure to evaluate the changes in liver function of all patients. Median follow-up duration was 11 months, with interquartile range from 6.5 to 18 months.
Technical and clinical successes were achieved in 18 of 19 patients (94.7%). Complete GV obliteration was confirmed using post-procedural CT scans in these 18 patients. The median interval from the procedure to the CT scan was 4 days. Only partial thrombus of GV was observed in 2 patients after 3 days or 1 week from the procedure, but complete thrombosis and obliteration were verified in follow-up CT after 1 month. Even a large varix with waist diameter of 18 mm and maximal diameter of 27 mm was successfully obliterated by deploying a 22 mm vascular plug to the waist without the need for complementary coil embolization.
Plug-assisted retrograde transvenous obliteration failed in 1 patient due to incomplete filling of the GV with the gelfoam slurry (Fig. 2). The patient had impaired liver function (Child Pugh classification B) and concomitant hepatocellular carcinoma with a history of endoscopic glue injection 7 weeks before the procedure due to the gastric variceal hemorrhage. Pre-procedural CT scan taken before the endoscopic injection clearly delineated the GV and GRS. Although the posterior gastric vein and left gastric vein were sufficiently visualized during the gelfoam embolization, the embolic material did not completely fill the upper portion of gastric varix. The patient was admitted through our emergency department with hematemesis 4 months after the PARTO. Recent gastric variceal hemorrhage was revealed in endoscopic evaluation and emergent endoscopic hemostasis was performed. One year later, the patient received liver transplantation. Therefore, the technical and clinical success rate for the PARTO was 94.7% (18/19 patients).
Supplementary embolization with 12 mm × 0.035-inch platinum coil (Nester, Cook) was necessary in 3 patients (15.8%) to ensure complete occlusion of efferent GRS.
Five patients showed small collateral veins during gelfoam embolization. In regard to the collateral veins, 4 patients showed pericardiacophrenic veins, and 1 patient had an inferior phrenic vein collateral. These small collaterals could be occluded with the injection of gelfoam slurry, and additional embolization was not carried out in any patients.
Four of 19 (21.1%) patients had minimal contrast leakage caused by the rupture of small collaterals during the gelfoam embolization. Nevertheless, filling of the GV continued slowly and embolization was technically successful. They did not experience clinical sign of hemorrhage or hemoglobin level drop after the procedure. On post-procedural CT scan, none showed retroperitoneal hematomas or fluid collection.
The median procedure time was 61 minutes, with interquartile range from 34.5 to 95.5 minutes. Shortening of procedure time from approximately 100 to 40 minutes throughout our study reflects the importance of the operator's experiences in PARTO. In 1 patient with left-sided inferior vena cava (IVC), the GRS could be approached directly through the left-sided IVC and the procedure time could be reduced (30 minutes).
Acute complications that occurred within 72 hours after the procedure were as follows: fever (n = 2), fever and hypotension (n = 2; one diagnosed adrenal insufficiency), and transient microscopic hematuria (n = 3). Two patients had fever (body temperature, 38℃) a few hours after the procedure. Though clinical focus of the fever was not clear in these patients, antipyretic drugs were administered to alleviate the symptom and their body temperatures were normalized on the next day. Two patients experienced fever and hypotension (body temperature 39℃, with blood pressure of 83/39 mm Hg, and 89/40 mm Hg, respectively), which were managed with fluid resuscitation. One patient recovered normal blood pressure within hours, but the blood pressure of the other patient fluctuated despite appropriate fluid therapy. In order to determine the cause of fluctuating blood pressure, low-dose adrenocorticotropic hormone test was conducted and adrenal insufficiency was confirmed in this patient. Oral hydrocortisone therapy with an initial dose of 30 mg per day (20 mg in morning and 10 mg in evening) was given and gradually tapered off for 3 months. The patient recovered completely after the completion of oral hydrocortisone therapy. Three patients (15.8%) manifested microscopic hematuria for 1–2 days after the PARTO procedure. Since hematuria resolved spontaneously in all these patients and post-procedural CT scans revealed no evidence of systemic thrombosis including renal vein thrombosis, the transient microscopic hematuria seemed to be idiopathic and clinically insignificant.
Post-procedural CT scans showed newly developed small amount of ascites in 5 of 18 (27.8%) successfully treated patients. Post-procedural CT scans of 6 patients who already had ascites before the procedure revealed increased amounts of ascites, but the increased volumes were not large enough to require drainage procedure. Thus, total 11 out of 19 patients (58%) showed newly developed or increased amount of ascites. Three patients showed small ectopic varices including paraumbilical, rectal varices and inferior mesocaval shunt.
Among post-procedural endoscopy of 10 patients, GV completely resolved in 3 patients, and improved in 5 patients. However, any endoscopic change was observed in 2 patients. Of these 2 patients, 1 patient was confirmed total thrombosis of GV on CT images conducted 4 days after our procedure, which was on the day that the endoscopy was performed. The other patient with technical failure, showed only partial thrombus in GV on the 1 month post-procedure follow-up CT scan. Diagnostic endoscopy performed 4 months later confirmed persistent GV and stigmata of gastric variceal bleeding. Endoscopic evaluation revealed newly developed EV in 1 of 18 patients who were successfully treated with PARTO. Four of 9 patients who already had EVs before the procedure experienced aggravation. As a result, total 5 out of 19 patients (26.3%) confirmed newly developed or aggravated EV by endoscopy. Two of these 4 patients had esophageal bleedings and 1 patient died of massive hemorrhage 7 months after PARTO. The other patient was successfully treated with endoscopic variceal ligation (EVL). Remaining 3 patients underwent prophylactic EVLs, even though they did not experience esophageal variceal bleeding.
Laboratory data from Wilcoxon signed-rank test revealed that international normalized ratio, total bilirubin and albumin levels were significantly improved after the procedure (p = 0.019, 0.000, and 0.004, respectively).
During follow-up period, recurrent gastric variceal hemorrhage was not reported among 18 well-treated patients. Four patients died during follow-up period due to liver failure (n = 3, 2 within 2 months, 1 within 6 months after the PARTO) and EV bleeding which occurred 7 months after the procedure (n = 1).
In our study, PARTO showed a high technical success rate (18/19; 94.7%). There was no recurrent gastric variceal hemorrhage, and complete GV obliteration was confirmed by post-procedural CT scan in all technically succeeded patients. These results were consistent with the good technical and clinical success rate of PARTO reported in the previous 2 studies (1314). Although conventional BRTO is reportedly effective in controlling gastric variceal hemorrhage (112), PARTO has several advantages over conventional BRTO. First, there is no risk of balloon rupture and subsequent pulmonary embolism, which can be fatal. Second, dose limitation of sclerosants is not an obstacle for PARTO, because gelfoam slurry is used instead. All patients with whom we had technically success showed complete obliteration of GV by single session. Prior studies on conventional BRTO reported high technical and clinical successes, but in some studies, repeated BRTO procedures were necessary for complete obliteration of large varies (9161718). Moreover, gelfoam is safer embolic material than ethanolamine oleate, which is recommended with 4000 units of haptoglobin to prevent renal failure (19). Gelfoam is also a more familiar embolic material to interventional radiologists because it is used in various procedures throughout the body. Third, PARTO does not require a long post-procedural bed-rest with the indwelling balloon catheter and full monitoring. Even though balloon dwelling time varies greatly from 30 minutes to 24 hours among studies, cases with short indwelling time tend to show low obliteration rate, so long indwelling time is preferred in cases with large varices for complete obliteration (1220).
We used a guiding catheter in some patients to deploy a plug in the narrowest portion of the GRS that is proximal to GV and apart from the left renal vein confluence. This could reduce the plug size, embolic volume, and procedure time. Considering the complication of systemic venous thrombosis after BRTO with reported incidence of 15% (21), we also anticipated that our method could reduce the risk of systemic venous thrombosis by preventing the plug from contacting left renal vein.
We used not only a vascular plug, but also additional coils for the complete obstruction of efferent flow in 3 patients. To overcome the size limitation of PARTO, Lee et al. (22) recently introduced microcoils instead of a vascular plug to occlude the GRS efferent flow. The study used 2 microcatheters, 1 for GRS embolization with the gelfoam pledgets and the other for blocking the GRS. Both CARTO and our PARTO are effective for the obliteration of GV, but it takes longer to block GRS with microcoils than with a vascular plug (mean procedure time 2.8 hours vs. our median procedure time 61 minutes). By using both vascular plug and coil, we could overcome the weak points of both procedures. Varices with large GRS can be occluded completely in a shorter procedure time. For very small GRS, however, it could be hard to pass 4 Fr catheter beside deployed vascular plug even when a minimum sized plug is used. CARTO or conventional BRTO could be superior in such a small GRS. Further study is required to verify the suitability of each procedure for these small GRSs.
For the 1 failed case, previous histoacryl injection to GV is suspected to be a culprit. Exact cause is uncertain, because PARTO was successful for the other patient with prior endoscopic glue injection. The injected histoacryl could possibly have blocked the GRS pathway, and there is a risk of later development of another shunt, such as gastrocaval shunt. Further study is required to clarify the effect of prior histoacryl injection to the technical failure of PARTO.
During the injection of gelfoam slurry, all collateral veins were occluded spontaneously. Because of the risk of gelfoam embolization to pulmonary arteries though the collateral veins, we gradually injected gelfoam pledgets under fluoroscopic guidance. Contrast media was injected in between to confirm the occlusion of these collaterals. Small amount of gelfoam could be circulated via these systemic venous connections, however, none of the patients had symptoms or signs of pulmonary embolism or systemic arterial embolism.
Minimal leakage of contrast solution to retroperitoneum reported in 4 patients was probably due to the rupture of small collateral. Leakage of contrast solution is not a common complication of conventional BRTO, but 2 previous studies reported contrast extravasation in 1 of 43 patients and 3 of 56 patients (2324). Contrast leakage may be attributed to rupture of small collateral veins caused by pressurized injection of the gelfoam pledgets, although the actual pressure was low. Further study is needed to investigate whether PARTO using gelfoam pledgets is associated with increased incidence of rupture of small collateral veins.
The effectiveness of emergency BRTO for treating ruptured GV was reported previously, but repeated procedures were required in some of the patients (2526). One-session PARTO procedure was sufficient for treating both hemostasis and obliteration of GV in our 2 patients. A previous study indicated that 25% of patients experience re-bleeding after histoacryl sclerotherapy of active gastric variceal hemorrhage (27). Therefore, PARTO could be an alternative method for preventing recurrent hemorrhage in patients with active gastric variceal bleeding.
Although post-procedural adrenal insufficiency had not been previously reported as a complication of BRTO, this complication can be explained anatomically. Since the GRS joins left adrenal vein before it meets the left renal vein, it is possible to occlude left adrenal vein during the BRTO procedure. Nevertheless, this complication is rarely symptomatic, probably because the remaining contralateral adrenal gland compensates for the shortage of the hormone. Adrenal insufficiency is a possible complication of the PARTO in certain susceptible individuals, and this complication should be suspected and treated in patients with the consistent clinical symptoms.
Aggravation of EV is one of the drawbacks of BRTO and also of the PARTO. Combining TIPS with BRTO is currently tried. Some of the post-BRTO complications including aggravation of EV and ascites, were attributed to the increased portal flow. In terms of hemodynamics, TIPS could alleviate portal hypertension caused by BRTO. Saad et al. (28) reported protective value of combined TIPS against the development of hydrothorax/ascites. Protective effect against esophageal variceal worsening was not evaluated in this study, however, it might work theoretically. Further studies are required for determining factors for patient selection and the optimal timing for combined or sequential TIPS.
Significant laboratory improvement after PARTO is concordant with previous studies on the conventional BRTO (129). We only evaluated short term change, i.e., within 1 week, and this laboratory improvement may largely reflect the effect of hemostasis and conservative management. However, long term (3 years) hepatic function improvement after conventional BRTO is previously reported (30), and PARTO is expected to show similar favorable effect.
In conclusion, PARTO is a technically feasible, safe, and effective treatment for gastric variceal hemorrhage in patients with portal hypertension.
Figures and Tables
Fig. 1
72-year-old female with GV and GRS.
A. 9 Fr TIPS sheath is placed in left renal vein and tip is in orifice to GRS. Contrast injection reveals GRS with waist (black arrow). Small inferior phrenic collateral vein (black arrowhead) is also seen. B. Vascular plug (arrow) is placed at waist of GRS. With gelfoam slurry injection, inferior phrenic collateral vein is spontaneously occluded and GV is completely filled with gelfoam mixture. C. Pre-procedural CT scan shows GV (asterisk). D. Complete thrombosis (asterisk) of GV is reported on post-procedural CT scan 4 days after PARTO. CT = computed tomography, GRS = gastrorenal shunt, GV = gastric varix, PARTO = plug-assisted retrograde transvenous obliteration, TIPS = transjugular intrahepatic portosystemic shunt
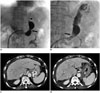
Fig. 2
58-year-old male with previous endoscopic histoacryl injection.
A. Injected glue (asterisk) is demonstrated in upper portion of GV. B. Gelfoam embolization is performed sufficiently until left gastric vein (white arrow) is visualized. C. GV (asterisk) is delineated on pre-procedural CT scan, which is performed 1 week before endoscopic glue injection and 8 weeks before PARTO procedure. Concomitant HCC (white arrow) is observed in left lateral lobe of liver, although it was not clearly visualized on this hepatic venous phase image. Post-ablation area (black arrow) is seen in right lobe of liver. D. Post-procedural CT scan reveals residual GV (black arrows) and previously injected endoscopic glue (black asterisk). Amount of ascites is increased after procedure. CT = computed tomography, GV = gastric varix, HCC = hepatocellular carcinoma, PARTO = plug-assisted retrograde transvenous obliteration
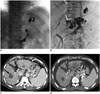
Table 1
Summary of Patient Characteristics

References
1. Saad WE, Sabri SS. Balloon-occluded retrograde transvenous obliteration (BRTO): technical results and outcomes. Semin Intervent Radiol. 2011; 28:333–338.
2. de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis. 2001; 5:645–663.
3. Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992; 16:1343–1349.
4. Lee CH, Lee JH, Choi YS, Paik SW, Sinn DH, Lee CY, et al. [Natural history of gastric varices and risk factors for bleeding]. Korean J Hepatol. 2008; 14:331–341.
5. Rosołowski M, Hartleb M, Marek T, Milewski J, Linke K, Wallner G, et al. Therapeutic and prophylactic management of bleeding from oesophageal and gastric varices - recommendations of the Working Group of the National Consultant for Gastroenterology. Prz Gastroenterol. 2014; 9:63–68.
6. Triantafyllou M, Stanley AJ. Update on gastric varices. World J Gastrointest Endosc. 2014; 6:168–175.
7. Saad WE, Darcy MD. Transjugular intrahepatic portosystemic shunt (TIPS) versus balloon-occluded retrograde transvenous obliteration (BRTO) for the management of gastric varices. Semin Intervent Radiol. 2011; 28:339–349.
8. Park SJ, Chung JW, Kim HC, Jae HJ, Park JH. The prevalence, risk factors, and clinical outcome of balloon rupture in balloon-occluded retrograde transvenous obliteration of gastric varices. J Vasc Interv Radiol. 2010; 21:503–507.
9. Hirota S, Matsumoto S, Tomita M, Sako M, Kono M. Retrograde transvenous obliteration of gastric varices. Radiology. 1999; 211:349–356.
10. Lee JY, Moon SH, Lee SM, Kim HT, Uh S, Kim YH, et al. A case of noncardiogenic pulmonary edema by ethanolamine oleate. Korean J Intern Med. 1994; 9:125–127.
11. Uchibori S. [Pulmonary circulatory disturbance following endoscopic injection sclerotherapy]. Nihon Kyobu Shikkan Gakkai Zasshi. 1993; 31:833–839.
12. Patel A, Fischman AM, Saad WE. Balloon-occluded retrograde transvenous obliteration of gastric varices. AJR Am J Roentgenol. 2012; 199:721–729.
13. Gwon DI, Ko GY, Yoon HK, Sung KB, Kim JH, Shin JH, et al. Gastric varices and hepatic encephalopathy: treatment with vascular plug and gelatin sponge-assisted retrograde transvenous obliteration--a primary report. Radiology. 2013; 268:281–287.
14. Gwon DI, Kim YH, Ko GY, Kim JW, Ko HK, Kim JH, et al. Vascular plug-assisted retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy: a prospective multicenter study. J Vasc Interv Radiol. 2015; 26:1589–1595.
15. Tajiri T, Yoshida H, Obara K, Onji M, Kage M, Kitano S, et al. General rules for recording endoscopic findings of esophagogastric varices (2nd edition). Dig Endosc. 2010; 22:1–9.
16. Akahoshi T, Tomikawa M, Kamori M, Tsutsumi N, Nagao Y, Hashizume M, et al. Impact of balloon-occluded retrograde transvenous obliteration on management of isolated fundal gastric variceal bleeding. Hepatol Res. 2012; 42:385–393.
17. Ninoi T, Nishida N, Kaminou T, Sakai Y, Kitayama T, Hamuro M, et al. Balloon-occluded retrograde transvenous obliteration of gastric varices with gastrorenal shunt: long-term follow-up in 78 patients. AJR Am J Roentgenol. 2005; 184:1340–1346.
18. Fukuda T, Hirota S, Sugimura K. Long-term results of balloon-occluded retrograde transvenous obliteration for the treatment of gastric varices and hepatic encephalopathy. J Vasc Interv Radiol. 2001; 12:327–336.
19. Hashizume M, Kitano S, Yamaga H, Sugimachi K. Haptoglobin to protect against renal damage from ethanolamine oleate sclerosant. Lancet. 1988; 2:340–341.
20. Cho SK, Shin SW, Lee IH, Do YS, Choo SW, Park KB, et al. Balloon-occluded retrograde transvenous obliteration of gastric varices: outcomes and complications in 49 patients. AJR Am J Roentgenol. 2007; 189:W365–W372.
21. Cho SK, Shin SW, Do YS, Park KB, Choo SW, Kim SS, et al. Development of thrombus in the major systemic and portal veins after balloon-occluded retrograde transvenous obliteration for treating gastric variceal bleeding: its frequency and outcome evaluation with CT. J Vasc Interv Radiol. 2008; 19:529–538.
22. Lee EW, Saab S, Gomes AS, Busuttil R, McWilliams J, Durazo F, et al. Coil-assisted retrograde transvenous obliteration (CARTO) for the treatment of portal hypertensive variceal bleeding: preliminary results. Clin Transl Gastroenterol. 2014; 5:e61.
23. Shimoda R, Horiuchi K, Hagiwara S, Suzuki H, Yamazaki Y, Kosone T, et al. Short-term complications of retrograde transvenous obliteration of gastric varices in patients with portal hypertension: effects of obliteration of major portosystemic shunts. Abdom Imaging. 2005; 30:306–313.
24. Sonomura T, Ono W, Sato M, Sahara S, Nakata K, Sanda H, et al. Three benefits of microcatheters for retrograde transvenous obliteration of gastric varices. World J Gastroenterol. 2012; 18:1373–1378.
25. Sonomura T, Ono W, Sato M, Sahara S, Nakata K, Sanda H, et al. Emergency balloon-occluded retrograde transvenous obliteration of ruptured gastric varices. World J Gastroenterol. 2013; 19:5125–5130.
26. Choi YH, Yoon CJ, Park JH, Chung JW, Kwon JW, Choi GM. Balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding: its feasibility compared with transjugular intrahepatic portosystemic shunt. Korean J Radiol. 2003; 4:109–116.
27. Khawaja A, Sonawalla AA, Somani SF, Abid S. Management of bleeding gastric varices: a single session of histoacryl injection may be sufficient. Eur J Gastroenterol Hepatol. 2014; 26:661–667.
28. Saad WE, Wagner CC, Lippert A, Al-Osaimi A, Davies MG, Matsumoto AH, et al. Protective value of TIPS against the development of hydrothorax/ascites and upper gastrointestinal bleeding after balloon-occluded retrograde transvenous obliteration (BRTO). Am J Gastroenterol. 2013; 108:1612–1619.
29. Saad WE, Wagner CC, Al-Osaimi A, Bliebel W, Lippert A, Davies MG, et al. The effect of balloon-occluded transvenous obliteration of gastric varices and gastrorenal shunts on the hepatic synthetic function: a comparison between Child-Pugh and model for end-stage liver disease scores. Vasc Endovascular Surg. 2013; 47:281–287.
30. Kumamoto M, Toyonaga A, Inoue H, Miyakoda K, Morita Y, Emori K, et al. Long-term results of balloon-occluded retrograde transvenous obliteration for gastric fundal varices: hepatic deterioration links to portosystemic shunt syndrome. J Gastroenterol Hepatol. 2010; 25:1129–1135.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download