Abstract
Objective
We aimed to evaluate the efficacy and safety of a newly developed, partially retrievable flow-diverter (the FloWise) in an elastase-induced rabbit aneurysm model.
Materials and Methods
We developed a partially retrievable flow diverter composed of 48 strands of Nitinol and platinum wire. The FloWise is compatible with any microcatheter of 0.027-inch inner diameter, and is retrievable up to 70% deployment. The efficacy and safety of the FloWise were evaluated in the elastase-induced rabbit aneurysm model. The rate of technical success (full coverage of aneurysm neck) and assessment of aneurysm occlusion and stent patency was conducted by angiograms and histologic examinations at the 1-month, 3-month, and 6-month follow-up. The patency of small arterial branches (intercostal or lumbar arteries) covered by the FloWise were also assessed in the 5 subjects.
Results
We attempted FloWise insertion in a total of 32 aneurysm models. FloWise placement was successful in 31 subjects (96.9%). Two stents (6.2%) were occluded at the 3-month follow-up, but there was no evidence of in-stent stenosis in other subjects. All stented aneurysms showed progressive occlusion: grade I (complete aneurysm occlusion) in 44.4% and grade II (aneurysm occlusion > 90%) in 55.6% at 1 month; grade I in 90% and II in 10% at 3 months; and grade I in 90% and II in 10% at 6 months. All small arterial branches covered by the FloWise remained patent.
Endovascular coiling is considered one of the standard treatments for intracranial aneurysms. The most critical drawback of coiling is a higher aneurysm recurrence rate, as compared to clipping, particularly in wide-necked or large aneurysms. The flow-diverter stent was developed to overcome this drawback, and has produced superior results to conventional coiling for large aneurysms. However, the currently available flow-diverter stent is not retrievable once deployment is initiated, and shows foreshortening of > 50%. Thus, we developed a new flow-diverter stent that is both partially retrievable and shows less foreshortening. The purpose of this study was to evaluate the efficacy and safety of the newly developed flow-diverter (the FloWise, Angiovention, Seoul, Korea) in the elastase-induced rabbit aneurysm model.
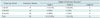
The study was approved by the Institutional Review Board for animal experiments. The FloWise is composed of 36 strands of 0.0012-inch Nitinol wire and 12 strands of 0.0012-inch platinum wire. These 48 strands are braided and heat-treated in the expanded configuration. After deployment from the delivery system, the FloWise expands to cover the neck of the aneurysm, forming a high-coverage mesh of approximately 33% up to 41% by area, with a radioopacity similar to that of the Pipeline embolization device (Covidien, Irvine, CA, USA) (Fig. 1A, B). The FloWise is attached to a flexible delivery wire, which has radiopaque end markers, and is packaged in an introducer sheath. The stent-contact portion of the delivery wire is coated with silicon so that it grips the FloWise, which allows for re-sheathing at any point prior to 70% deployment (Fig. 1). This packaged device can be loaded into standard microcatheters with at least a 0.027-inch inner diameter. It is pushed through the microcatheter and deployed by a combination of microcatheter withdrawal and forward pressure on the delivery wire. The FloWise undergoes approximately 38% shortening when deployed completely. Table 1 showed the comparative properties of the FloWise with 2 other types of commercially available flow diverters (Pipeline, Covidien, Irvine, CA, USA; Silk, Balt Extrusion, Montmorency, France).
Elastase-induced aneurysms were created in 31 New Zealand white rabbits, as previously reported (12). Aneurysms were allowed to mature for at least 4 weeks before insertion of the FloWise. Five days prior to FloWise insertion, each rabbit received daily dual-antiplatelet therapy (aspirin 10 mg/kg and clopidogrel 10 mg/kg per os). Under general anesthesia, a surgical cut-down of the right femoral artery was performed, and a 5-Fr sheath was inserted. A 5-Fr Envoy guide catheter (Coddman Neurovascular, Miami Lakes, FL, USA) was placed into the aortic arch, and a digital subtraction angiogram (DSA) was obtained to confirm aneurysm creation, measure aneurysm neck and dome diameter, and measure the parent artery at points proximal and distal to the aneurysm. Heparin 300 units was administered intravenously, and the guiding catheter was placed into the orifice of innominate artery. Next, a 0.027-inch microcatheter (Rebar, Covidien) was navigated over a 0.014-inch microguidewire into the right subclavian artery, distal to the aneurysm. The guidewire was removed, and the FloWise advanced and deployed across the aneurysm neck. Subsequently, the microcatheter was also removed, and DSA obtained at the aortic arch. In 5 animals, an additional FloWise was placed in the thoracic and abdominal aorta, across the origin of at least 1 pair of intercostal or lumbar arteries. Following the removal of the guiding catheter and femoral sheath, the femoral artery was ligated. Antiplatelet therapy was continued for either 30 days (1-month follow-up group) or 90 days (3-month and 6-month follow-up groups). The animals were followed for 1 month (n = 9), 3 months (n = 12), or 6 months (n = 10). At follow-up, DSA was obtained at the aortic arch and descending aorta. The animals were then sacrificed with a lethal injection of pentobarbital. Harvested aneurysms and stented aortic segments were immediately fixed in 10% neutral buffered formalin.
Follow-up angiograms assessed aneurysm occlusion using a 3-point scale: grade I for complete aneurysm occlusion; grade II for > 90% occlusion of the aneurysm sac; and grade III for < 90% occlusion of the aneurysm sac, as previously reported (34). Patency of the parent artery and its branches, including intercostal or lumbar arteries covered by the FloWise, was also assessed.
After routine tissue processing, the fixed samples were embedded in paraffin. Samples were then sectioned at 5 µm to 6 µm, and stained with hematoxylin and eosin. Axial sections were taken from the proximal, mid, and distal portions of the aneurysm's parent artery. Morphometric measurements were performed using digital planimetry with a calibrated microscope system. Neointimal thickness was measured as the distance from the inner surface of each stent strut to the luminal border, as previously reported (34).
Statistical analysis was performed using IBM SPSS version 20 (IBM Corp., Armonk, NY, USA). Continuous variables were presented as a mean ± standard deviation, and categorical variables as a number and percent. Comparisons of aneurysm size and parent artery neointimal thickness between the follow-up groups was performed using one-way analysis of variance. Statistical significance was determined at p < 0.05 for a 95% confidence interval.
Aneurysm neck and sac sizes were 3.3 ± 1.1 mm and 6.5 ± 2.4 mm for the 1 month, 4.3 ± 1.4 mm and 7.2 ± 2.6 mm for the 3 month, and 3.2 ± 1.2 mm and 6.9 ± 2.2 mm for the 6 month follow-up groups, respectively. There were no significant differences in neck or sac size among the 3 groups with p value of 0.923 and 0.843, respectively.
The comparison among the FloWise and commercially available flow diverters were summarized in the Table 1 and the overall follow-up results were summarized in Table 2. Deployment of the FloWise was successful in 31 (96.9%) of 32 cases. In the only failed case, the distal end of the device had herniated into the aneurysm sac due to tortuous and fusiform change of the parent artery near the end of the deployment. Of the 31 cases in which the FloWise was implanted bridging the aneurysm neck, 2 stents (6.2%) were occluded; in both cases, the parent artery showed a fusiform appearance with severe tortuosity (Fig. 2). The remaining 29 aneurysms were available for follow-up: 9 in the 1 month, 10 in the 3 month, and 10 in the 6 month groups. All stented aneurysms showed progressive occlusion: grade I (complete occlusion) in 4 aneurysms (44.4%) (Fig. 3) and grade II (near occlusion) in 5 aneurysms (55.6%) at 1 month; grade I in 9 (90%) (Fig. 4) and grade II in 1 (10%) at 3 months; and grade I in 9 (90%) (Fig. 5) and grade II in 1 (10%) at 6 months follow-up, respectively. There were no occurrences of in-stent stenosis.
The mean diameter of the lumbar artery origin was 1.2 ± 0.3 mm. No cases of lumbar artery occlusion were noted on follow-up angiography (Fig. 5A) or scanning electron microscopic examination (Fig. 6). The endothelial cells covering the stent were contiguous with the endothelium of the parent artery (Fig. 6).
In the 1-month group, histology revealed complete covering of the aneurysm neck by the neointima along stent struts in 4 of 9 aneurysms (Fig. 3), and partial covering in the remaining 5 aneurysms, which showed grade II occlusion on follow-up angiogram. The 3-month group showed complete covering of the aneurysm neck by the neointima (Fig. 4) in 9 of 10 aneurysms, and partial covering in the remaining 1, which showed grade II occlusion on follow-up angiogram. The 6-month group showed complete covering of the aneurysm neck by the neointima (Fig. 5) in 9 of 10 aneurysms and partial covering in the remaining 1, which showed grade II occlusion on follow-up angiogram. Thus, histologic results were in exact concordance with those of follow-up angiograms.
The neointimal thickness of the parent artery was a mean of 0.12 ± 0.09 mm for the one month, 0.11 ± 0.08 mm for the 3 month, and 0.15 ± 0.12 mm for the 6 month groups, respectively. There were no significant differences in neointimal thickness among the follow-up groups.
This study presents the efficacy and safety data of a newly developed flow-diverter stent, the FloWise, in the elastase-induced rabbit aneurysm model. The FloWise was highly trackable, with a radioopacity similar to that of the Pipeline. It had radial force and metal coverage capacities greater than that of the Pipeline or the Silk, while showing less foreshortening than both devices. In-vivo, the FloWise demonstrated a 100% complete or near occlusion rate. In addition, it was possible to re-sheath the FloWise up till 70% deployment, allowing for greater flexibility with device re-positioning.
As in previous reports, all small lumbar arteries covered by the FloWise remained patent, likely due to runoff blood flow. However, as perforator artery occlusion has been reported after Pipeline implantation in humans, the patency of perforator arteries with the FloWise is not always guaranteed in humans.
In this in-vivo experiment, there were 2 cases of stent occlusion on follow-up angiography. Both cases occurred in markedly tortuous, fusiform-appearing parent arteries, which was likely due to leakage of elastase into the parent artery during aneurysm creation. This appearance made it difficult to cross the aneurysm neck with the microcatheter, resulting in twisting, compaction of struts with increased stent diameter, and incomplete apposition of the FloWise to the parent artery wall; this in turn might have caused in-stent thrombosis and eventual stent occlusion (Fig. 6).
Several types of flow-diverters are used for intracranial aneurysm occlusion in clinical practice, particularly for treatment of large and giant aneurysms (5678). According to recent meta-analysis, the complete occlusion rate was 76% overall, with 80% for small, 74% for large, and 76% for giant aneurysms. The procedure-related morbidity and mortality rates were 5% and 4%, respectively. The rate of postoperative subarachnoid hemorrhage was 3%, and rate of intraparenchymal hemorrhage was 3%. The perforator infarction rate was 3%, with significantly lower rates of perforator infarction in patients with anterior circulation versus posterior circulation (9). Although the risk of procedure-related morbidity and mortality is not negligible, the treatment of intracranial aneurysms with flow-diverters is feasible and effective, yielding high complete occlusion rates when used for large, giant, uncoilable, or failed aneurysms (5910).
Until now, the Pipeline, the only flow-diverter that is available in Korea, has several drawbacks. First, because of its high cost, reimbursement is given only to aneurysms ≥ 15 mm in diameter. Second, it is mainly composed of a Co-Cr alloy, so that the self-expanding radial force is often insufficient for proper positioning of the device to the vessel wall, especially in a tortuous parent artery. As described, the FloWise demonstrates increased radial force, better metal coverage, and less foreshortening than both the Pipeline and the Silk (Table 1). Additionally, it allows for re-sheathing, making repositioning possible until 70% stent deployment. Thus, FloWise may be easier to use than currently available flow-diverters.
This study had several limitations. We did not directly compare the FloWise and other commercially available flow-diverters. However, because we performed in-vivo experiments under the same conditions as in previous in-vivo reports (34), it is reasonable to conclude that the FloWise would function similarly to commercially available flow diverters in clinical practice.
In conclusion, a newly developed, partially retrievable flow-diverter, the FloWise, might provide a safe and effective method of aneurysm occlusion, as evaluated in an elastase-induced rabbit aneurysm model.
Figures and Tables
Fig. 1
FloWise flow-diverter and its delivery system.
A. FloWise flow-diverter. B. Spot images of FloWise (left) with Pipeline (right). C. Delivery system of FloWise. Stent-contact portion of delivery wire is coated with silicon to hold stent, which makes it possible to re-sheath FloWise until < 70% deployed.

Fig. 2
Occlusion of FloWise.
A. Aortic angiogram shows tortuous and fusiform change of right subclavian artery close to elastase-induced aneurysm, possibly due to leakage of elastase into right subclavian artery. B. 4.5 × 20 mm FloWise stent was implanted from subclavian origin to right vertebral artery origin. Because subclavian artery appears tortuous and fusiform with 5.5 mm in fusiform segment, Flowise was deformed and enlarged with compaction of stent struts at fusiform segment (arrows). Angiogram just after Flowise implantation shows markedly decreased inflow into aneurysm sac due to more increased pore density and decreased porosity by stent strut compaction. C. However, at 3-month follow-up angiogram, Flowise is mildly shortened and occluded at fusiform segment of strut compaction and deformation. Arrowheads indicate proximal and distal ends of FloWise.
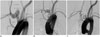
Fig. 3
Representative angiograms showing grade I aneurysm occlusion, and photomicrograph from 1-month, 3-month, and 6-month groups.
A. Pre-stent angiogram showing saccular aneurysm. B. Angiogram just after FloWise insertion showing contrast medium filling only up to neck of aneurysm. C. One-month follow-up angiogram shows complete occlusion (grade I) of aneurysm sac. D. Photomicrograph (× 15) shows neointimal formation completely covering aneurysm.
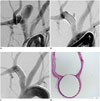
Fig. 4
Representative angiograms and photomicrograph from 3-month group.
A. Pre-stent angiogram showing saccular aneurysm. B. Angiogram just after FloWise insertion shows contrast filling neck to less than third of aneurysm sac. C. 3-month follow-up angiogram shows complete occlusion (grade I) of aneurysm sac. D. Photomicrograph (× 15) shows neointimal formation completely covering aneurysm neck.
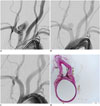
Fig. 5
Representative angiograms and photomicrograph from 6-month group.
A. Pre-stent angiogram showing saccular aneurysm. B. Angiogram just after FloWise insertion shows contrast filling neck to < third of aneurysm sac. C. 6-month follow-up angiogram shows complete occlusion (grade I) of aneurysm sac. D. Photomicrograph (× 15) shows neointimal formation completely covering aneurysm neck.
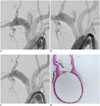
Fig. 6
Representative angiogram and scanning electron microscopies after FloWise insertion into descending aorta.
A. 6 month follow-up angiogram shows patent intercostal and lumbar arteries. B. Electron microscopy (× 20) shows patent lumen of lumbar artery covered by struts of FloWise. C. Electron microscope (× 50) shows that tissue covering struts at orifice of lumbar artery is contiguous with that of device at aorta.

Table 1
Properties of FloWise and Two Commercially Available Flow-Diverters

References
1. Altes TA, Cloft HJ, Short JG, DeGast A, Do HM, Helm GA, et al. 1999 ARRS Executive Council Award. Creation of saccular aneurysms in the rabbit: a model suitable for testing endovascular devices. American Roentgen Ray Society. AJR Am J Roentgenol. 2000; 174:349–354.
2. Krings T, Möller-Hartmann W, Hans FJ, Thiex R, Brunn A, Scherer K, et al. A refined method for creating saccular aneurysms in the rabbit. Neuroradiology. 2003; 45:423–429.
3. Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A new endoluminal, flow-disrupting device for treatment of saccular aneurysms. Stroke. 2007; 38:2346–2352.
4. Kallmes DF, Ding YH, Dai D, Kadirvel R, Lewis DA, Cloft HJ. A second-generation, endoluminal, flow-disrupting device for treatment of saccular aneurysms. AJNR Am J Neuroradiol. 2009; 30:1153–1158.
5. Lylyk P, Miranda C, Ceratto R, Ferrario A, Scrivano E, Luna HR, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: the Buenos Aires experience. Neurosurgery. 2009; 64:632–642. discussion 642-643quiz N6
6. Lubicz B, Collignon L, Raphaeli G, Pruvo JP, Bruneau M, De Witte O, et al. Flow-diverter stent for the endovascular treatment of intracranial aneurysms: a prospective study in 29 patients with 34 aneurysms. Stroke. 2010; 41:2247–2253.
7. Tähtinen OI, Manninen HI, Vanninen RL, Seppänen J, Niskakangas T, Rinne J, et al. The silk flow-diverting stent in the endovascular treatment of complex intracranial aneurysms: technical aspects and midterm results in 24 consecutive patients. Neurosurgery. 2012; 70:617–623. discussion 623-624
8. Yu SC, Kwok CK, Cheng PW, Chan KY, Lau SS, Lui WM, et al. Intracranial aneurysms: midterm outcome of pipeline embolization device--a prospective study in 143 patients with 178 aneurysms. Radiology. 2012; 265:893–901.
9. Brinjikji W, Murad MH, Lanzino G, Cloft HJ, Kallmes DF. Endovascular treatment of intracranial aneurysms with flow diverters: a meta-analysis. Stroke. 2013; 44:442–447.
10. Becske T, Kallmes DF, Saatci I, McDougall CG, Szikora I, Lanzino G, et al. Pipeline for uncoilable or failed aneurysms: results from a multicenter clinical trial. Radiology. 2013; 267:858–868.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download