Abstract
Chordoid glioma is a rare low grade tumor typically located in the third ventricle. Although a chordoid glioma can arise from ventricle with tumor cells having features of ependymal differentiation, intraventricular dissemination has not been reported. Here we report a case of a patient with third ventricular chordoid glioma and intraventricular dissemination in the lateral and fourth ventricles. We described the perfusion MR imaging features of our case different from a previous report.
Chordoid glioma of the third ventricle, a rare neuroepithelial tumor of the central nervous system, mostly originates from the anterior part of the third ventricle. Chordoid glioma is classified as grade II tumor according to 2007 World Health Organization (WHO) classification of brain tumors (1). Approximately 85 cases of chordoid glioma have been reported in English literatures since its first report in 1998 (2). Chordoid glioma is typically located in the third ventricle or suprasellar region. It often extends into the hypothalamus (12). Although chordoid glioma is located within the ventricular system, intraventricular seeding or metachronous tumors along the ventricular wall has not been reported. All reported cases of chordoid glioma have been solitary mass so far. Herein, we present the first case of chordoid glioma arising from the third ventricle with multifocal intraventricular seeding in the lateral and fourth ventricles. We also described perfusion MR imaging features of this case different from a previous report. This case report was approved by our institutional ethics committee. The requirement of patient informed consent was waived because this was a retrospective investigation.
A 34-year-old man presented with a history of 10-day headache. Neurological examination at the presentation was unremarkable. A head CT scan revealed a large high attenuated mass with lobulated margins and small calcifications in the anterior 3rd ventricle (Fig. 1A). Additional small hyper-attenuated masses along the ependymal surface of both lateral ventricles were also found. A large area of peritumoral edema was noted in the left frontal periventricular white matter. A brain MRI revealed a 3.1 × 4.2 × 4.5 cm well defined multi-lobulated tumor in the anterior third ventricle and suprasellar region displacing the right lateral ventricle superiorly (Fig. 1B). The mass showed iso-signal intensity on both T1- and T2-weighted images relative to the brain cortex. No intratumoral hemorrhage was observed on susceptibility-weighted imaging. The mass demonstrated homogenous strong enhancement after intravenous injection of gadolinium. Postcontrast T1-weighted imaging also demonstrated additional multiple enhancing masses along the ependymal surface of lateral ventricles, the fourth ventricle, and in the bilateral foramen of Luschka. Obstructive hydrocephalus was also noted. Perfusion MRI showed markedly elevated cerebral blood volume (CBV) within the tumors on CBV map (Fig. 1C). The ratio of maximum tumor CBV relative to white matter was calculated using the following equation: maximum rCBV = maximum CBV tumor/maximum CBV white matter. The maximum CBV was obtained using a circular region of interest with a diameter of 10 mm from each main tumor and the adjacent right frontal white matter. The maximum rCBV was 6.95.
The patient first underwent a tumor removal surgery for the daughter mass in the left lateral ventricle through a left frontal transcortical approach. At surgery, a hard multi-lobulated and encapsulated mass was found to be attached to the ependymal wall. The tumor was gross totally resected. Two months following the first surgery, a second operation was performed to remove the main tumor in the third ventricle. The patient underwent tumor removal surgery via a bifrontal craniotomy and a transcallosal interhemispheric approach. At surgery, a hard whitish mass filling the anterior part of the third ventricle was found to be firmly attached to the hypothalamus. The tumor had multiple arterial feeders on its surface. There were also several small daughter tumors along the ventricular surface. The main tumor was also gross totally resected.
Histopathologic examination for both tumor specimens obtained from each surgery showed identical histologic features. On histology, these tumors consisted of clusters and cords of oval-to-polygonal epithelioid cells with eosinophilic cytoplasm in a mucinous stroma (Fig. 1D). There was no evidence of mitosis or microvascular proliferation on light microscopy. Immunohistochemical analysis revealed that these tumor cells were diffusely positive for glial-fibrillary acid protein (GFAP), CD 34, and vimentin. In addition, they were focally positive for epithelial membrane antigen (EMA) and S-100 protein (Fig. 1E, F) with a Ki-67 labeling index of less than 0.5%. Their pathologic diagnosis was chordoid glioma. At 45 months follow-up, the patient was doing well without any evidence of tumor recurrence in the third or lateral ventricles on follow-up MRI. Follow-up MRI also showed no changes in the size of small enhancing tumors in the fourth ventricle or foramen of Luschka.
Chordoid glioma is a rare low grade neuroepithelial tumor with both glial and chordoid features. The tumor tends to occur in adult women (a female to male ratio of 2 to 1) at mean age of 45 years (range, 5-72 years) (3). Clinical symptoms including headache, nausea, vomiting, visual disturbances, confusion, lethargy, urinary incontinence, ataxia, hypothyroidism, diabetes insipidus, weight loss or weight gain, and behavioral disturbances due to intracranial hypertension, hydrocephalus, compression of the hypophysis, optic chiasm, and hypothalamus by the tumor (123).
Chordoid glioma is typically arising in the region of the anterior third ventricle/hypothalamus. There have been a few reports regarding the unusual locations of chordoid glioma such as juxtaventricular white matter and thalamus (4). In contrast to previous reports, our case of chordoid glioma had a large main tumor in the third ventricle with multiple smaller masses in the lateral and fourth ventricles, suggesting intraventricular dissemination of the third ventricular tumor rather than concomitant tumor occurrence. Given the location of the tumor, there is a possibility of intraventricular seeding of choroid glioma which has not been previously reported. The number of reported cases is very small. Therefore, the true biological behavior of this tumor remains to be established (4).
Our case supports the idea that chordoid glioma might be derived from ependymal cells or tanycytes. Several studies have shown that tumor cells of chordoid glioma have features of ependymal differentiation on electron microscopy such as basal lamina, microvilli, and intermediate filaments (5). Tanycytes are believed to be the primitive progenitor cells in the phylogenetic development of ependymal cells and glial cells. They are primarily present in the circumventricular organs with predominance in the anterior portion of the third ventricle (6). Brain tumors arising from the ependymal lining of the ventricles are likely to have cerebrospinal fluid (CSF) seeding. For example, CSF seeding has been seen in about 8% to 33% of patients with ependymoma at presentation (7). Although intraventricular seeding of diffuse WHO grade II glioma is rare, it can occur during follow-up period after incomplete resections of primary tumor (8).
On imaging studies, chordoid gliomas are solid, ovoid in shape, well circumscribed, and located in the anterior third ventricle and/or suprasellar region. At CT, most tumors are hyperdense on unenhanced scan and enhanced homogenously after injection of contrast agent. At MRI, chordoid gliomas have isosignal intensity on T1-weighted images and slightly hypersignal intensity on T2-weighted images. They have strong homogenous enhancement following gadolinium injection (3). Intratumoral calcification or cyst has been observed in rare cases. However, intratumoral hemorrhage or necrosis has not been reported. There has been only one case report describing the perfusion MR imaging features of chordoid glioma. Grand et al. (9) have reported that the CBV of the tumor is not elevated compared to that of white matter. In addition, there is no microvascular proliferation on histology (9). The relative maximum CBV ratio was 1 in the study of Grand et al. (9). However, our case showed a markedly elevated maximum CBV within the tumor. The relative maximum CBV ratio was 6.95 in our case, suggesting that this tumor entity might be composed of heterogeneous subgroups. However, T1-weighted leakage and T2- and/or T2*-weighted imaging residual effects were not corrected in our case. Thus, rCBV of the tumor might be overestimated in our case.
On histopathology, chordoid gliomas had characteristic appearance consisting of cords and clusters of epithelioid cells with eosinophilic cytoplasm and relatively uniform round to oval nuclei embedded in mucinous stroma (123). High grade features such as mitoses or necrosis were absent or rare. Immunohistochemically, chordoid gliomas were strongly and diffusely positive for GFAP, vimentin, CD 34, and focally positive for EMA and cytokeratin. CD 34 is positive in 93% of reported cases (3). Therefore, CD 34 can be used to differentiate chordoid glioma from chordoid meningioma, pilocytic astrocytoma, and ependymoma (3). Our case was also diffusely positive for GFAP, CD 34, vimentin, and focally positive for EMA and S-100 protein.
Surgical resection of the tumor is considered as the treatment of choice. Recurrence is rare following gross total resection. However, gross total resection is often difficult given its location in the deep structure of brain and its proximity to critical neurovascular structures. High morbidity and mortality rates after surgical resection have been reported. Therefore, a more conservative treatment approach other than surgical resection is warranted (10). In our case, there was no tumor recurrence at post-resection sites. In addition, there was no change in remained tumor in the fourth ventricle on follow-up MRI after 45 months of gross total resection, confirming the low grade nature of this tumor. Using chemotherapy for chordoid glioma has not been reported.
In conclusion, we described the first case of chordoid glioma in the third ventricular with intraventricular dissemination in the lateral and fourth ventricles. Although chordoid glioma is a low grade tumor, the tumor can have elevated CBV on perfusion MR imaging. Our case suggests that chordoid glioma is composed of heterogeneous subgroups. Further studies are needed to understand the biological behavior of this rare tumor.
Figures and Tables
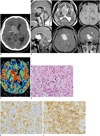
Fig. 1
Chordoid glioma in 34-year-old man.
A. Axial unenhanced CT showing hyperattenuated mass compressing frontal horn of right lateral ventricle. Note small calcification (arrow) in periphery of mass. B. Sagittal T1-weighted (upper left panel) and axial T2-weighted (upper central panel) images showing isointense lobulated mass (arrows) relative to cerebral cortex in anterior third ventricle. Axial susceptibility-weighted image (upper right panel) showing no evidence of intratumoral hemorrhage. Sagittal (lower left panel), axial (lower central panel), and coronal (lower right panel) post-contrast T1-weighted images showing strong enhancing main tumor with lobulated margin (arrows) in anterior part of third ventricle and smaller enhancing masses (arrowheads) along wall of lateral ventricles and fourth ventricle. C. CBV map of perfusion MRI showing elevated CBV within tumor (arrows) in third ventricle. D. Photomicrograph of hematoxylin and eosin stained slide showing solid cellular components composed of clusters and cords of epithelioid tumor cells (arrows) within variable mucinous stroma (original magnification × 400). CBV = cerebral blood volume E, F. Photomicrographs of immunostained slides for GFAP (E) and CD 34 (F) showing diffuse and strong expression in tumor cells (dark yellow and brown colors) (original magnification × 400). GFAP = glial-fibrillary acid protein
References
1. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007; 114:97–109.
2. Tanboon J, Aurboonyawat T, Chawalparit O. A 29-year-old man with progressive short term memory loss. Brain Pathol. 2014; 24:103–106.
3. Vanhauwaert DJ, Clement F, Van Dorpe J, Deruytter MJ. Chordoid glioma of the third ventricle. Acta Neurochir (Wien). 2008; 150:1183–1191.
4. Kim JW, Kim JH, Choe G, Kim CY. Chordoid glioma: a case report of unusual location and neuroradiological characteristics. J Korean Neurosurg Soc. 2010; 48:62–65.
5. Pasquier B, Péoc'h M, Morrison AL, Gay E, Pasquier D, Grand S, et al. Chordoid glioma of the third ventricle: a report of two new cases, with further evidence supporting an ependymal differentiation, and review of the literature. Am J Surg Pathol. 2002; 26:1330–1342.
6. Agarwal S, Stevenson ME, Sughrue ME, Wartchow EP, Mierau GW, Fung KM. Features of intraventricular tanycytic ependymoma: report of a case and review of literature. Int J Clin Exp Pathol. 2014; 7:3399–3407.
7. Ambekar S, Ranjan M, Prasad C, Santosh V, Somanna S. Fourth ventricular ependymoma with a distant intraventricular metastasis: report of a rare case. J Neurosci Rural Pract. 2013; 4:Suppl 1. S121–S124.
8. Alvarez de Eulate-Beramendi S, Rigau V, Taillandier L, Duffau H. Delayed leptomeningeal and subependymal seeding after multiple surgeries for supratentorial diffuse low-grade gliomas in adults. J Neurosurg. 2014; 120:833–839.
9. Grand S, Pasquier B, Gay E, Kremer S, Remy C, Le Bas JF. Chordoid glioma of the third ventricle: CT and MRI, including perfusion data. Neuroradiology. 2002; 44:842–846.
10. Desouza RM, Bodi I, Thomas N, Marsh H, Crocker M. Chordoid glioma: ten years of a low-grade tumor with high morbidity. Skull Base. 2010; 20:125–138.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download