Abstract
Objective
The purpose of this study was to demonstrate the usefulness of digital subtraction cystography to identify communicating holes between a spinal extradural arachnoid cyst (SEAC) and the subarachnoid space prior to cyst removal and hole closure.
Materials and Methods
Six patients with SEAC were enrolled in this retrospective study. Digital subtraction cystography and subsequent CT myelography were performed for every patient. The presence and location of the communicating holes on cystography were documented. We evaluated the MRI characteristics of the cysts, including location, size, and associated spinal cord compression; furthermore, we reviewed cystographic images, CT myelograms, procedural reports, and medical records for analysis. If surgery was performed after cystography, intraoperative findings were compared with preoperative cystography.
Results
The location of the communicating hole between the arachnoid cyst and the subarachnoid space was identified by digital subtraction cystography in all cases (n = 6). Surgical resection of SEAC was performed in 4 patients, and intraoperative location of the communicating hole exactly corresponded to the preoperative identification.
Spinal extradural arachnoid cysts (SEACs) are rare and can cause spinal cord compression (1). They are thought to arise from congenital defects in the dura mater and 116protrusions of the arachnoid herniating out through the defects; many authors have speculated that a one-way valve mechanism causes intermittent increased pressure within the extradural cyst, leading to expansion (23). SEACs almost always communicates with the spinal subarachnoid space through a small dural defect (4). Symptomatic cysts are primarily treated with resection of the cyst wall and complete obliteration of the communicating hole between the cyst and the subarachnoid space after laminectomy of the affected vertebrae (1567). Preoperative identification of the communicating hole between the cyst and the subarachnoid space is important to tightly close the dural defect (7).
Several attempts have been made to locate the communicating hole between the cyst and subarachnoid space using special magnetic resonance imaging (MRI) techniques (489). To our knowledge, no previous studies have addressed digital subtraction cystography to detect the communicating hole of SEAC. In this study, we presented several cases of SEAC in which the opening was identified by digital subtraction cystography. Furthermore, we introduced the usefulness of digital subtraction cystography in preoperative evaluation.
This retrospective study was approved by our Institutional Review Board and informed consent for enrollment was waived. From November 2014 to April 2015, 6 patients with SEAC were referred from the Neurosurgery Department to the Radiology Department for spinal cystography. Four females and 2 males were included in this study, with a mean age of 36.3 years (range 20-51). All patients had symptoms associated with the cyst, including lower leg paresthesia (n = 5), low back pain (n = 5), lower extremity weakness (n = 2), and urinary difficulty (n = 1) (Table 1). One patient (patient 5) had undergone surgery on an arachnoid cyst 11 years prior. Despite the surgical cystectomy, she visited our hospital for the recurrence of cyst.
Before the procedure, informed consent was acquired from all patients. Cystography was conducted under fluoroscopic guidance in an angiography suite (Siemens Artis Zee System, Erlangen, Germany). Intravenous conscious sedation or general anesthesia was not necessary. The patient was placed in the prone position on the fluoroscopy table, and we targeted the region of the cyst based on prior spinal MRI. After sterile preparation using 2% chlorhexidine and local anesthesia with 1% lidocaine, a 22-gauge 12-cm spinal needle was gently advanced into the extradural arachnoid cyst via a midline interlaminar approach under fluoroscopic guidance. After confirming the proper needle placement within the arachnoid cyst using both the frontal and lateral fluoroscopic projections, about 10 mL of cerebrospinal fluid (CSF) was slowly aspirated in order to relieve compression of the spinal cord by the cyst. Digital subtraction cystography was performed after a test injection of contrast medium (Omnipaque 300 [iohexol, 300 mg I/mL]; GE Healthcare, Milwaukee, WI, USA) to verify that the spinal needle tip was within the cyst. While patient respiration was suspended, 3-4 mL of contrast medium was injected by hand at a rate of approximately 0.5 mL/s, and a digital subtraction acquisition was performed in the lateral projection at a film rate of 1 frame per second. A dural defect was defined as clear contrast flow from the cyst into the subarachnoid space on digital subtraction cystography. The frontal plane of digital subtraction cystography was also performed to verify the accuracy of the dural defect site. We injected 10 mL of contrast medium into each patient. Spinal computed tomography (CT) scans were performed to obtain CT myelograms in the supine position within 30 minutes of spinal cystography.
All patients underwent preprocedural MR imaging of the spine, including sagittal and axial sequences. We evaluated imaging characteristics of the cysts, including craniocaudal location, longest diameter of the cyst, and associated spinal cord compression or myelopathy. Digital subtraction cystographic images, CT myelograms, procedural reports, and electronic medical records were reviewed for analysis. The location of the communicating hole on cystographic images was documented as follows: right or left side and pedicle, infrapedicle, or disc level. If the opening was located between the upper endplate of the vertebral body and the inferior margin of the pedicle on frontal and lateral fluoroscopic images, the lesion was classified as pedicle level. Lesions below the inferior margin of the pedicle were classified as infrapedicle level, and openings located between the endplates of the vertebrae were classified as disc level (Fig. 1). We assessed MRI and CT myelograms for any findings indicative of communicating holes. In patients who underwent surgical treatment, intraoperative findings regarding fistulas and pathologic results of the cystic walls were analyzed.
All cysts were located below the mid-thoracic level; 3 (50%) were in the thoraco-lumbar junction, 2 (33.3%) were in the lower thoracic level, and 1 (16.7%) was in the upper lumbar level (Table 2). All cysts were located in the posterior region of the spinal canal. The longest diameter of the cysts was an average of 88.5 mm with a range of 52 to 140 mm. All lesions had iso-intense signal, as compared to CSF on all sequences, and were separated from the thecal sac by a thin septa. In 1 patient (patient 4), a hyperintense focus on T2-weighted MRI was evident in the compressed spinal cord, suggestive of compressive myelopathy (Fig. 2). Epidural fat obliteration, bony erosion and scalloping, and spinal canal widening were seen in all patients. We identified additional smaller SEACs in 2 patients (patients 1 and 6), with no continuity between the cysts. In patient 1, the smaller cyst did not show contrast filling on CT myelogram (Fig. 3), possibly due to severe cord compression by the cyst and interruption of upward CSF flow. We did not observe any sign indicative of a communicating hole, such as a turbulent flow void within the cyst, on preoperative MRI and CT myelograms.
There were no significant complications associated with cystography except for mild back pain during the procedure. The sites of the communicating holes in all patients were visualized using digital subtraction cystography as active extravasation of contrast medium from the cyst into the thecal sac (Fig. 2). The locations of the communicating holes were summarized in Table 2. Four patients underwent surgery after cystography, and the intra-operative locations of the holes exactly corresponded with those predicted using cystography. The openings of the cysts were closed intraoperatively with subtotal cyst wall resections. Only 1 patient (patient 1) underwent laminectomy of more than 2 vertebral levels. The pathology revealed arachnoid tissue in the cystic wall, consistent with spinal arachnoid cyst. After the surgery, symptoms were improved in all 4 patients, with side effects of only mild back pain or a residual tingling sensation.
Spinal extradural arachnoid cyst is diagnosed based on characteristic MRI findings of a posterior extradurally located expansile cystic lesion with signal characteristics identical to CSF on T1- and T2-weighted images. CT myelography is better for confirming communication between the cyst and the subarachnoid space through the dural defect and thus allows accurate diagnosis of these lesions (13). Partial cyst wall resection and closure of the communicating hole yield excellent results in terms of avoiding spinal instability and malalignment after complete cyst resection (410). Accurate preoperative identification of the location of the communicating hole is necessary to successfully close the communicating hole with minimal laminectomy and cyst resection (9).
The results of our study indicated that conventional MRI and CT myelography usually fail to detect the location of the communicating hole, as it only comprises a small dural defect. Several studies have used special MR imaging techniques such as cine-MRI and time-spatial labeling inversion pulse (T-SLIP) MRI to locate the defect between the cyst and the subarachnoid space (489). Cine-MRI was introduced to detect the communication site by showing pulsatile turbulent flow voiding (8); however, this method cannot always detect the dural defect (910). Funao et al. (10) reported that communicating holes were revealed in only 2 of 12 patients with arachnoid cyst on cine-MRI. T-SLIP MRI is another technique to visualize CSF flow (4), but it is not useful if the flow through the communicating hole is not directly posterior. If the flow through the communicating hole is posterolateral or lateral, a suitable scanning plane and inversion pulse position should be selected (4). In addition, a small hole might not be visualized by T-SLIP MRI because of the lower resolution of the cinematic MRI (4).
In this study, digital subtraction cystography reliably and directly detected the opening and flow between the cyst and the thecal sac. The opening was located by cystography in every case, and surgery confirmed the preoperative identification. Cystography is an invasive procedure, as compared with MR imaging. It can cause post-dural puncture headache and further compression of the spinal cord due to injection of contrast media into the cyst. We aspirated about 10 mL of CSF from the cysts prior to injecting contrast medium in order to relieve cord compression. Conventional myelography is not as invasive and is easily used in daily practice (11). Cystography is similar to myelography and does not require a special technique. We observed no complications except for mild back pain during the procedure. Direct cystography might be impossible for anteriorly located SEACs due to the difficulty of needle puncture without cord injury. However, most SEACs are located dorsally and allow a posterior needle approach (12).
There were several limitations to this study. Only 6 patients were included because of the low prevalence of this disease. Secondly, pathological confirmation of arachnoid cyst was not performed in 2 patients (patients 3 and 5) because surgery was not performed after cystography. The neurologic symptoms of these patients were not severe, and the degree of spinal canal stenosis was mild on MRI and CT myelograms. The neurosurgeon and the patients decided to delay surgery and continue conservative management. While the diagnosis of SEAC was based on MRI and CT myelography, other cystic tumors were excluded by characteristic imaging findings. In addition, 1 patient (patient 5) underwent a previous operation on the cyst 11 years prior, and pathology confirmed the diagnosis. Lastly, long-term follow-up was not conducted after surgery. More investigation is needed for long-term clinical outcomes of selective laminectomy with fistula closure.
In conclusion, fluoroscopic-guided direct cystography accurately identifies the presence and location of dural defects in patients with SEAC. Preoperative digital subtraction cystography is very helpful for detection of the communicating hole between the cyst and the subarachnoid space.
Figures and Tables
Fig. 2
47-year-old man with low back pain and left leg paresthesia of 2 month duration (patient 4).
A. T2-weighted sagittal MRI shows spinal extradural cyst compressing spinal cord at T11 and T12 levels. Focal hyperintense spot (arrow) is seen on spinal cord at T11 level, suggesting compressive myelopathy. B. Lateral digital subtraction cystographic image shows pooling of contrast in arachnoid cyst and contrast flow (arrow) from cyst to thecal sac, site of communicating hole. C. Frontal digital subtraction cystographic image shows dark linear focus (arrow), demonstrating contrast flow into thecal sac through dural defect at left pedicle level of T12 vertebra. Tip of spinal needle is located between pedicles. D. Axial CT myelogram obtained immediately after cystography shows contrast filling in extradural cyst on dorsal aspect of spinal cord. Dural sac is filled with contrast media, suggesting presence of communication between cyst and subarachnoid space.

Fig. 3
20-year-old woman with low back pain and bilateral leg weakness of 8 month duration (patient 1).
A. Preoperative T2-weighted sagittal MRI shows 2 extradural cysts with septation on dorsal aspect of spinal cord. Larger cyst compresses spinal cord, especially at T7-8 and T8-9 disc levels. B. Frontal cystographic image with direct puncture at T7-8 disc level shows large contrast-filled cyst. Dark spot (arrow) at right pedicle level of T9 vertebra is suspected as focal dural defect. C. Opening is better demonstrated as small focus (arrow) on digital subtraction cystographic image, and contrast flow (arrowheads) is seen in thecal sac. D. Sagittal CT myelogram shows contrast filling within cyst, compressing spinal cord. Contrast fills thecal sac only below larger cyst, while upper small cyst is not filled with contrast media. E. Postoperative T2-weighted MRI 39 days after surgery demonstrates disappearance of lower larger cyst seen on cystogram, and residual smaller extradural cyst is recognized at T7 level.
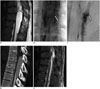
Table 1
Summary of Patient Data

Table 2
MRI Findings and Outcome of Procedures

References
1. Liu JK, Cole CD, Kan P, Schmidt MH. Spinal extradural arachnoid cysts: clinical, radiological, and surgical features. Neurosurg Focus. 2007; 22:E6.
2. McCrum C, Williams B. Spinal extradural arachnoid pouches. Report of two cases. J Neurosurg. 1982; 57:849–852.
3. Congia S, Coraddu M, Tronci S, Nurchi G, Fiaschi A. Myelographic and MRI appearances of a thoracic spinal extradural arachnoid cyst of the spine with extra- and intraspinal extension. Neuroradiology. 1992; 34:444–446.
4. Ishibe T, Senzoku F, Ikeda N, Kamba Y, Mikawa Y. Detection of the communicating hole(s) of spinal extradural arachnoid cysts using time-spatial labeling inversion pulse magnetic resonance imaging. Spine (Phila Pa 1976). 2014; 39:E1394–E1397.
5. Rimmelin A, Clouet PL, Salatino S, Kehrli P, Maitrot D, Stephan M, et al. Imaging of thoracic and lumbar spinal extradural arachnoid cysts: report of two cases. Neuroradiology. 1997; 39:203–206.
6. Doita M, Nishida K, Miura J, Takada T, Kurosaka M, Fujii M. Kinematic magnetic resonance imaging of a thoracic spinal extradural arachnoid cyst: an alternative suggestion for exacerbation of symptoms during straining. Spine (Phila Pa 1976). 2003; 28:E229–E233.
7. Tomii M, Mizuno J, Takeda M, Matsushima T, Itoh Y, Numazawa S, et al. Thoracolumbar extradural arachnoid cyst--three surgical case reports. Neurol Med Chir (Tokyo). 2013; 53:129–133.
8. Neo M, Koyama T, Sakamoto T, Fujibayashi S, Nakamura T. Detection of a dural defect by cinematic magnetic resonance imaging and its selective closure as a treatment for a spinal extradural arachnoid cyst. Spine (Phila Pa 1976). 2004; 29:E426–E430.
9. Miyamoto M, Kim K, Matsumoto R, Isobe M, Isu T. Utility of preoperative magnetic resonance imaging myelography for identifying dural defects in patients with spinal extradural arachnoid cysts: case report. Neurosurgery. 2006; 59:E941. discussion E941.
10. Funao H, Nakamura M, Hosogane N, Watanabe K, Tsuji T, Ishii K, et al. Surgical treatment of spinal extradural arachnoid cysts in the thoracolumbar spine. Neurosurgery. 2012; 71:278–284. discussion 284
11. Yoshida H, Takai K, Taniguchi M. Leakage detection on CT myelography for targeted epidural blood patch in spontaneous cerebrospinal fluid leaks: calcified or ossified spinal lesions ventral to the thecal sac. J Neurosurg Spine. 2014; 21:432–441.
12. Qi W, Zhao L, Fang J, Chang X, Xu Y. Clinical characteristics and treatment strategies for idiopathic spinal extradural arachnoid cysts: a single-center experience. Acta Neurochir (Wien). 2015; 157:539–545.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download