Abstract
Objective
To evaluate T2 relaxation time change using axial T2 mapping in a rabbit degenerated disc model and determine the most correlated variable with histologic score among T2 relaxation time, disc height index, and Pfirrmann grade.
Materials and Methods
Degenerated disc model was made in 4 lumbar discs of 11 rabbits (n = 44) by percutaneous annular puncture with various severities of an injury. Lumbar spine lateral radiograph, MR T2 sagittal scan and MR axial T2 mapping were obtained at baseline and 2 weeks and 4 weeks after the injury in 7 rabbits and at baseline and 2 weeks, 4 weeks, and 6 weeks after the injury in 4 rabbits. Generalized estimating equations were used for a longitudinal analysis of changes in T2 relaxation time in degenerated disc model. T2 relaxation time, disc height index and Pfirrmann grade were correlated with the histologic scoring of disc degeneration using Spearman's rho test.
Results
There was a significant difference in T2 relaxation time between uninjured and injured discs after annular puncture. Progressive decrease in T2 relaxation time was observed in injured discs throughout the study period. Lower T2 relaxation time was observed in the more severely injured discs. T2 relaxation time showed the strongest inverse correlation with the histologic score among the variables investigated (r = -0.811, p < 0.001).
Several recent studies have focused on prevention of disc degeneration or regeneration of the disc with growth factors (123), and stem cells (45). Annular puncture model in the rabbit is a popular model of disc degeneration, because it is a relatively cheap, simple method to produce disc degeneration rapidly (6). Typically, disc degeneration model is made by surgical stabbing of annulus fibrosus under anesthesia. Percutaneous annular puncture model guided by fluoroscopy (7) or CT (8) was recently introduced as a minimally invasive model to produce disc degeneration, which allows researchers to study the safety and efficacy of new therapeutic interventions.
Usually non-invasive methods to assess the degenerated disc include measuring the disc height on plain radiograph, and classification of the degenerated disc with MR T2 grading system by morphology and signal intensity (910). Usefulness of MR T2 mapping of human disc is well known and the calculation of representative T2 relaxation time in the region of interest (ROI) is widely used as quantitative measurement of disc degeneration (111213). T2 relaxation time of human intervertebral disc has a strong correlation with water content of the disc and patient's age, and disc degeneration grading by MR T2 images (11121314). MR T2 mapping of intervertebral disc in the rabbit was recently reported by Sun et al. (15) and used as a noninvasive method to monitor the change of disc degeneration after interventions in sagittal or coronal planes (161718). However, the sagittal or coronal plane limits the area of intervertebral discs in the field of view more than the axial plane. The single mid sagittal or mid coronal scan of discs may also limit detection of early degeneration in eccentric location (19).
The purpose of this study was to evaluate T2 relaxation time change of the rabbit degenerated disc model by axial T2 mapping, and determine the most correlated variable to histologic score among T2 relaxation time, disc height index (DHI) and Pfirrmann grade.
Eleven New Zealand white rabbits (male; 5 months old; mean weight, 3.27 kg; weight range, 2.56-4.00 kg) were used in this study, which was approved by the Institutional Animal Care and Use Committee. After anesthesia, lateral plain radiographs, baseline MR T2 sagittal scan and T2 map were obtained before annular puncture. Fluoroscopy-guided percutaneous annular puncture was done by posterolateral oblique approach. L6-7 discs were not included, because of probable effect by lumbosacral and sacroiliac junction. Annular puncture was done in 4 random lumbar discs in each rabbit causing 4 injuries of differing severity, i.e., single annular puncture with 23 G, 21 G, 18 G needle and multiple (3 times) annular punctures with 18 G needle. One lumbar disc in each rabbit was considered as a control. The levels of control and experimental discs were randomized in each rabbit to prevent bias that may occur by the level of the disc. Annular puncture was done until the needle tip reached center of the disc on fluoroscopic image (Fig. 1).
Imaging studies were done 3 times in 7 rabbits (group 1), before the annular puncture, and at 2 weeks and 4 weeks after the annular puncture. Additional 6 week imaging was done for the other 4 rabbits (group 2) to obtain enough cases of advanced disc degeneration.
After anesthesia, lumbar spine lateral radiograph was taken in all rabbits before percutaneous annular puncture to measure disc height using a VB Endura C-arm System (Philips Medial Systems, Shelton, CT, USA). MRI was performed using a MAGNETOM Trio Tim 3T MR System (Siemens, Forchheim, Germany). Sagittal T2-weighted images, including all lumbar vertebral discs, were acquired using a fast spin-echo sequence (pulse repetition time/echo time = 4090/101 ms, acquisition matrix = 512 × 245, echo train length = 19, field of view = 160 × 128 mm, pixel band width [BW] = 199 kHz, slice thickness = 1.5 mm, phase encoding [PE] direction = head to feet) for Pfirrmann grading of disc degeneration in all rabbits. Three axial images for T2 mapping (echo time 1-echo time 7 = 20-140 ms, acquisition matrix = 256 × 166, field of view = 120 × 120 mm, pixel BW = 106 kHz, slice thickness = 1 mm, PE direction = right to left) was obtained in each experimental and control discs. Extreme care was taken in locating every 2nd slice out of 3 slices with 1 mm thickness in the mid level of the disc, because the height of rabbit disc is only 1-2.5 mm. Inadequate localization may lead to underestimation of T2 relaxation time, by scanning eccentric axial slice of the disc.
Disc height was defined as the shortest dimension drawn between the mid points of adjacent endplates. Disc height measurement was done by 1 radiologist (5 year experience in musculoskeletal MR) in 2 separate sessions and averaged values were obtained. The relative disc height (RDH) compared to the control disc was used for DHI calculation, because the measured disc height may be influenced by the degree of muscle relaxation during anesthesia. RDH and DHI were calculated as follows:
For MR T2 grading of disc degeneration in lumbar intervertebral discs, a grade from 1 to 5 was assigned on the basis of Pfirrmann's (9) criteria by 1 radiologist (5 years experience of musculoskeletal MR) in 2 separate sessions. Final assignment was made in the third session for disagreed cases. Discs were classified as grade 1 if the nucleus pulposus and inner annulus fibrosus had normal height and bright signal intensity, grade 2 if the nucleus pulposus and inner annulus fibrosus has inhomogeneous signal intensity with or without horizontal band, grade 3 if the nucleus pulposus and inner annulus shows inhomogeneous gray intermediate signal intensity or slightly decreased height, grade 4 if the nucleus pulposus and annulus fibrosus shows intermediate to low signal intensity or moderately decreased height, and grade 5 if nucleus pulposus and annulus shows low signal intensity and collapsed disc space (Fig. 2).
For measurement of T2 relaxation time, 5 mm2 sized ROI was drawn manually in the center of nucleus pulposus in axial T2 map images that were automatically generated by MR system. All imaging analysis was done using an image work station, M-view PACS system (Infinitt Healthcare, Seoul, Korea).
After the final radiographs and MR images were obtained, the rabbits were sacrificed. Control and experimental discs were harvested for histologic studies. The mid sagittal section of disc with adjacent endplates were fixed in 10% neutral buffered formalin containing 10% cetylpyridinium chloride, decalcified in Cal-Ex II Fixative/Decalcifier (Fisher Scientific, Pittsburgh, PA, USA), paraffin-embedded, and sectioned to a 6 micrometer thickness. The sections were stained with hematoxylin and eosin. Histologic score was given by 1 radiologist, using the histologic grading scale (Table 1), developed by Masuda et al. (10), ranging from a normal disc with 4 points (1 point in 4 category) to a severely degenerated disc with 12 points (3 points in 4 category). Typical histologic finding of discs were shown in Figure 3.
Statistical analysis was performed with IBM SPSS 20.0 (IBM SPSS Statistics, Armonk, NY, USA). Generalized estimating equations were used for a longitudinal analysis of T2 relaxation time changes in degenerated disc model. Spearman's rho test was done for correlation of T2 relaxation time, DHI, and Pfirrmann grade with the histologic score.
Seven rabbits of group 1 underwent imaging studies, before and 2 weeks after annular puncture. Only 6 rabbits were available for imaging studies 4 weeks after annular puncture, because 1 rabbit died of unknown cause a day after 2 week imaging study. All 4 rabbits of group 2 underwent imaging studies before and 2 weeks, 4 weeks, and 6 weeks after annular puncture. Total 180 discs of serial imaging studies in 11 rabbits were included in this study.
T2 relaxation time value showed progressive decrease in injured discs over the study period (Fig. 4). The significant decrease of T2 relaxation time was observed at 2 weeks, 4 weeks, and 6 weeks after annular puncture in injured discs (p < 0.001). Also, significant decrease of T2 relaxation time was confirmed in all injured discs, when grouped analysis was done by severity of injury (p < 0.001 in all severity of injury). In contrast, no significant decrease in T2 relaxation time for the control discs was observed through the 6-week period (p = 0.744).
T2 relaxation time in disc models of variable strength injury, at 2 weeks, 4 weeks, and 6 weeks after annular puncture were shown in Table 2. T2 relaxation time values of the discs injured by 23 gauge single annular punctures were not significantly different from that of control discs at 2 weeks and 4 weeks (p = 0.796, 0.122, respectively). But T2 relaxation time value continued to decrease and was significantly different from that of control discs at 6 weeks after annular puncture (p = 0.005). More severely injured discs punctured by 21 gauge, 18 gauge needle single or multiple times showed significantly lower T2 value, as compared to that of control discs from 2 weeks and throughout the study period.
All vertebral discs in serial imaging studies were categorized as Pfirrmann grade 1 in baseline imaging study. Total 180 discs in all follow-up imaging were assigned as grade 1 (healthy, n = 99, including control discs and discs before annular puncture), grade 2 (n = 31), grade 3 (n = 25), grade 4 (n = 22), and grade 5 (severely degenerated, n = 0). The T2 relaxation time values in different Pfirrmann grades were listed in Table 3. Pfirrmann grades of rabbit disc models at the 2 weeks and 4 weeks time points were shown in Figure 5. Higher Pfirrmann grades were assigned in injured discs at 6 weeks, as compared to 4 weeks after the annular puncture. Pfirrmann grades also showed significant difference between injured discs and control disc with time effect (p < 0.001). However, there was no significant difference of DHI between injured disc and control discs over the 6-week period (p = 0.443).
T2 relaxation time showed the strongest inverse correlation with the histologic score with the correlation coefficient of -0.811 (p < 0.001). Pfirrmann grade also showed a fairly strong correlation (r = 0.770, p < 0.001) with the histologic score. However, there was a weak correlation between DHI and the histologic score (r = 0.394, p = 0.021).
The method of Masuda et al. (10) is widely used for the measurement of disc height. But there are several limitations to disc height measurement on plain radiographs. First, the rabbit must be under the same level of anesthesia, because the disc heights vary according to the depth of sedation and muscle relaxation. The plain radiograph has its own limitation in proper positioning to reveal the true disc height in every disc, because of coneshaped X-ray beam and different distances from object to X-ray detector. There may be errors of the disc height measurement on plain radiograph, because the vertebral endplates of rabbits are more concave and vertebral bodies have curved margin, which may cause low reproducibility. Finally and most importantly, early disc degeneration, that can show minimal or no height change, cannot be assessed by disc height measurement alone. DHI are calculated from RDH in this study to reduce bias from different depth of anesthesia and muscle relaxation, assuming that the depth of anesthesia affects disc height equally at all levels of lumbar spine by muscle relaxation and that height of control level does not change after the puncture of other discs. Nevertheless, DHI showed fair correlation to histologic score with r = 0.394, and the difference between injured discs and control discs were not significant in this study.
Pfirrmann et al. (9) introduced an MR grading system for disc degeneration, assessed by disc morphology and signal intensity on MR T2-weighted images. Pfirrmann grade is widely used in classifying disc degeneration; and early disc degeneration is assessable as grade 2 disc that does not show decrease of disc height, by change of signal intensity. However, it is also limited, because it is a semi-quantitative method and subjective modality that relies on the radiologists' vision. Also, the discs in the same grade of degeneration show wide variability of signal intensity and morphology. Furthermore, Pfirrmann grade has limitation of morphological analysis, which cannot assess biochemical and biomechanical properties of degenerated disc.
However, T2 mapping of degenerated disc gives information of water content and tissue integrity. Marinelli et al. (19) reported that T2 relaxation time was significantly correlated with water content of human cadaver nuclei. Subsequently, the same group reported that T2 relaxation times correlated with Pfirrmann grade in degenerated discs (20). Interestingly, for the 44 Pfirrmann grade 2 discs, the mean T2 relaxation time of nuclear region were decreased in aged persons, as compared to young adults. They suggested that decrease of T2 relaxation time is associated with decreasing glycosaminoglycan and water content.
In this study, the T2 relaxation times of nucleus pulposus in the rabbits agrees well with that of human intervertebral discs and rabbit models, in other published studies. Blumenkrantz et al. (12) reported that mean T2 relaxation times of human nucleus pulposus were grade 2 (92.3 ± 27.2 ms), grade 3 (59.5 ± 12.5 ms), grade 4 (59.6 ± 7.6 ms), and grade 5 (37 ms). Trattnig et al. (14) reported mean T2 relaxation times of human nucleus pulposus, in normal disc (Pfirrmann grade 1 and grade 2) as 128.5 ± 42.4 ms, and that of degenerated disc (Pfirrmann grade 3 and grade 4) as 78.5 ± 31.2 ms. Mean T2 relaxation times of rabbit nucleus pulposus in different Thompson grades were reported by Sun et al. (15) calculated in mid sagittal plane; grade 1 (121.6 ± 10.4 ms), grade 2 (87.5 ± 16.4 ms), grade 3 (64.2 ± 11.5 ms), and grade 4 (45.4 ± 7.9 ms). In this study, T2 relaxation times of nucleus pulposus were grade 1 (121.93 ± 26.87 ms), grade 2 (112.93 ± 20.03 ms), grade 3 (76.12 ± 24.55 ms), and grade 4 (53.63 ± 9.69 ms). The T2 relaxation time by axial T2 mapping in this study agrees with sagittal T2 mapping in the rabbit disc in previous studies.
T2 relaxation time measurement is now an emerging quantitative method to evaluate disc degeneration. Watanabe et al. (21) introduced a new classification of disc degeneration with axial T2 mapping. They showed that the conventional MR grading system with T2-weighted image shows larger difference of T2 value between grade 1 and grade 2 discs, but smaller difference of T2 value between grade 2 and 3, or grade 3 and 4. However, T2 relaxation time shows more uniform differences of T2 value between all grades of disc degeneration, and is more suitable for T2 based classification. Welsch et al. (13) cautiously suggested that quantitative T2 mapping might enable a diagnosis of longitudinal changes in the biochemical and biomechanical profile of a degenerated disc, which was demonstrated in our study. T2 relaxation time value of the injured disc showed progressive decrease over the study period, whereas no significant decrease in T2 relaxation time for the control discs was observed through the 6-week period (p > 0.05). Also, more severely injured discs showed more rapid decrease in T2 relaxation time. Finally, T2 relaxation time was most strongly correlated to histologic score (r = -0.811, p < 0.001) among the other variables in our study. The result of our study indicated that T2 relaxation time measurement is a valuable method to evaluate the disc degeneration process, non-invasively.
This study had several limitations. First, all radiological and histological assessments were done by 1 radiologist, who was blinded to other results, and intra-observer/inter-observer variability was not assessed. Second, 3 discs were excluded in T2 mapping, because of severe artifact. Moreover, the rabbit disc is very small and careful localization is needed to obtain proper mid axial scan, as compared to mid sagittal scan, without partial-volume effect and eccentric localization. Therefore, rabbits weighing ≥ 3 kg are suitable for axial T2 mapping. The sagittal scans are known to minimize partial volume effect, but the mid-sagittal scan cannot represent degeneration of whole disc, because early disc degeneration could be eccentric. Axial T2 mapping of nucleus pulposus should be more valuable for early eccentric disc degeneration. Third, there was no grade 5 disc in this study due to short total duration of the study (6 weeks) and disc degeneration may progress over time. Grade 5 disc is severely degenerated, with severe decreases in height. So the probability of having partial volume effect is increased in grade 5 discs, which were not shown in this study. But most of the studies on disc regeneration after a specific treatment usually target early disc degeneration and axial T2 mapping could be a feasible method to monitor disc degeneration or regeneration at early stages. Also, height loss can be less frequently seen in rabbits, because gravity has little effect on the spine of the rabbit than on that of the human.
In conclusion, quantitative axial T2 mapping of degenerated disc model in the rabbit is a valuable method to assess the disc degeneration process; in addition, T2 relaxation time showed the strongest correlation to histologic score, as compared to DHI and Pfirrmann Grading System.
Figures and Tables
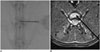 | Fig. 1Fluoroscopic guided annular puncture of lumbar intervertebral disc in rabbit.
A. Fluoroscopic image showing needle placement on anteroposterior projection. Note needle tip reaches middle 1/3 to ensure full thickness puncture of annulus fibrosus. B. Route of annular puncture is demonstrated by black arrow on axial MR T2-weighted image.
|
 | Fig. 2Pfirrmann grade 1 to grade 4 discs assessed with MR T2-weighted sagittal scan.
A. Grade 1 disc shows homogenous bright high signal intensity in nucleus pulposus and inner annulus fibrosus. B. Grade 2 disc shows inhomogeneous high signal intensity in nucleus pulposus with less distinct margin between nucleus pulposus and annulus fibrosus. C. Grade 3 disc shows intermediate signal intensity in nucleus pulposus and slightly decreased disc height. D. Grade 4 disc shows moderately decreased disc height and intermediate to low signal intensity in nucleus. Note focal disc herniation (open arrow) to central zone.
|
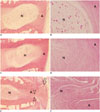 | Fig. 3Typical histologic grades of disc degeneration.Hematoxylin and eosin stained sections of normal (A and B, histologic score 4), moderately degenerated (C and D, histologic score 10), severely degenerated (E and F, histologic score 12) discs show obliteration of border between nucleus pulposus (N) and annulus fibrosus (A), more fibrous, less cellular nucleus pulposus with increasing severity. Note annular tear (open arrowheads) and disc herniation (open arrow) in severely degenerated disc as shown in E (A, C, E at 30 x and B, D, F at 200 x).
|
 | Fig. 4Mean T2 relaxation time at each time point.Significant decrease in T2 relaxation time for injured discs is observed with time (p < 0.001). Error bars represent standard deviation from mean.
|
 | Fig. 5Number of discs in different Pfirrmann grades of degenerative disc models are demonstrated in different columns according to severity of injury, 2 weeks and 4 weeks after annular puncture. |
Table 1
Definition of Histologic Grading Scale

Table 2
T2 Relaxation Time of Discs after Annular Puncture Injury

References
1. Masuda K. Biological repair of the degenerated intervertebral disc by the injection of growth factors. Eur Spine J. 2008; 17:Suppl 4. 441–451.
2. Chujo T, An HS, Akeda K, Miyamoto K, Muehleman C, Attawia M, et al. Effects of growth differentiation factor-5 on the intervertebral disc--in vitro bovine study and in vivo rabbit disc degeneration model study. Spine (Phila Pa 1976). 2006; 31:2909–2917.
3. Masuda K, Oegema TR Jr, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine (Phila Pa 1976). 2004; 29:2757–2769.
4. Yang H, Wu J, Liu J, Ebraheim M, Castillo S, Liu X, et al. Transplanted mesenchymal stem cells with pure fibrinous gelatin-transforming growth factor-beta1 decrease rabbit intervertebral disc degeneration. Spine J. 2010; 10:802–810.
5. Leung VY, Chan D, Cheung KM. Regeneration of intervertebral disc by mesenchymal stem cells: potentials, limitations, and future direction. Eur Spine J. 2006; 15:Suppl 3. S406–S413.
6. Kim KS, Yoon ST, Li J, Park JS, Hutton WC. Disc degeneration in the rabbit: a biochemical and radiological comparison between four disc injury models. Spine (Phila Pa 1976). 2005; 30:33–37.
7. Kwon YJ. A minimally invasive rabbit model of progressive and reproducible disc degeneration confirmed by radiology, gene expression, and histology. J Korean Neurosurg Soc. 2013; 53:323–330.
8. Zhou RP, Zhang ZM, Wang L, Huang MJ, Zheng XC, Cui YN, et al. Establishing a disc degeneration model using computed tomography-guided percutaneous puncture technique in the rabbit. J Surg Res. 2013; 181:e65–e74.
9. Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine (Phila Pa 1976). 2001; 26:1873–1878.
10. Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, et al. A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine (Phila Pa 1976). 2005; 30:5–14.
11. Perry J, Haughton V, Anderson PA, Wu Y, Fine J, Mistretta C. The value of T2 relaxation times to characterize lumbar intervertebral disks: preliminary results. AJNR Am J Neuroradiol. 2006; 27:337–342.
12. Blumenkrantz G, Zuo J, Li X, Kornak J, Link TM, Majumdar S. In vivo 3.0-tesla magnetic resonance T1rho and T2 relaxation mapping in subjects with intervertebral disc degeneration and clinical symptoms. Magn Reson Med. 2010; 63:1193–1200.
13. Welsch GH, Trattnig S, Paternostro-Sluga T, Bohndorf K, Goed S, Stelzeneder D, et al. Parametric T2 and T2* mapping techniques to visualize intervertebral disc degeneration in patients with low back pain: initial results on the clinical use of 3.0 Tesla MRI. Skeletal Radiol. 2011; 40:543–551.
14. Trattnig S, Stelzeneder D, Goed S, Reissegger M, Mamisch TC, Paternostro-Sluga T, et al. Lumbar intervertebral disc abnormalities: comparison of quantitative T2 mapping with conventional MR at 3.0 T. Eur Radiol. 2010; 20:2715–2722.
15. Sun W, Zhang K, Zhao CQ, Ding W, Yuan JJ, Sun Q, et al. Quantitative T2 mapping to characterize the process of intervertebral disc degeneration in a rabbit model. BMC Musculoskelet Disord. 2013; 14:357.
16. Cai F, Wu XT, Xie XH, Wang F, Hong X, Zhuang SY, et al. Evaluation of intervertebral disc regeneration with implantation of bone marrow mesenchymal stem cells (BMSCs) using quantitative T2 mapping: a study in rabbits. Int Orthop. 2015; 39:149–159.
17. Subhan RA, Puvanan K, Murali MR, Raghavendran HR, Shani S, Abdullah BJ, et al. Fluoroscopy assisted minimally invasive transplantation of allogenic mesenchymal stromal cells embedded in HyStem reduces the progression of nucleus pulposus degeneration in the damaged ntervertebral [corrected] disc: a preliminary study in rabbits. ScientificWorldJournal. 2014; 2014:818502.
18. Obata S, Akeda K, Imanishi T, Masuda K, Bae W, Morimoto R, et al. Effect of autologous platelet-rich plasma-releasate on intervertebral disc degeneration in the rabbit anular puncture model: a preclinical study. Arthritis Res Ther. 2012; 14:R241.
19. Marinelli NL, Haughton VM, Muñoz A, Anderson PA. T2 relaxation times of intervertebral disc tissue correlated with water content and proteoglycan content. Spine (Phila Pa 1976). 2009; 34:520–524.
20. Marinelli NL, Haughton VM, Anderson PA. T2 relaxation times correlated with stage of lumbar intervertebral disk degeneration and patient age. AJNR Am J Neuroradiol. 2010; 31:1278–1282.
21. Watanabe A, Benneker LM, Boesch C, Watanabe T, Obata T, Anderson SE. Classification of intervertebral disk degeneration with axial T2 mapping. AJR Am J Roentgenol. 2007; 189:936–942.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download