Abstract
Objective
To evaluate the relationship between response categories assessed by magnetic resonance imaging (MRI) or pathology and survival outcomes, and to determine whether there are prognostic differences among molecular subtypes.
Materials and Methods
We evaluated 174 patients with biopsy-confirmed invasive breast cancer who had undergone MRI before and after neoadjuvant chemotherapy, but before surgery. Pathology findings were classified as a pathologic complete response (pCR) or a non-pCR, and MRI findings were designated as a radiologic CR (rCR) or a non-rCR. We evaluated overall and subtype-specific associations between clinicopathological factors including the assessment categories and recurrence, using the Cox proportional hazards model.
Results
There were 41 recurrences (9 locoregional and 32 distant recurrences). There were statistically significant differences in recurrence outcomes between patients who achieved a radiologic or a pCR and patients who did not achieve a radiologic or a pCR (recurrence hazard ratio, 11.02; p = 0.018 and recurrence hazard ratio, 3.93; p = 0.022, respectively). Kaplan-Meier curves for recurrence-free survival showed that triple-negative breast cancer was the only subtype that showed significantly better outcomes in patients who achieved a CR compared to patients who did not achieve a CR by both radiologic and pathologic assessments (p = 0.004 and 0.001, respectively). A multivariate analysis found that patients who achieved a rCR and a pCR did not display significantly different recurrence outcomes (recurrence hazard ratio, 2.02; p = 0.505 and recurrence hazard ratio, 1.12; p = 0.869, respectively).
Neoadjuvant chemotherapy (NAC) is increasingly being used in patients with locally advanced breast cancer, since studies have shown that survival outcomes and locoregional control achieved with NAC are similar to those achieved with adjuvant chemotherapy (123). The use of NAC provides valuable information about the efficacy of experimental therapies and the tumor's response to standard therapies. This information may allow clinicians to change regimens based on the knowledge gained about NAC (45).
Many studies have reported that pathologic complete response (pCR) can be used as a surrogate endpoint for prediction of long-term clinical benefits such as disease-free survival and overall survival (67). Although pCR is the most commonly used endpoint in neoadjuvant trials, various definitions of pCR have been used by different investigators. Therefore, the survival outcomes of patients with pCR have been controversial. This has led to confusion about interpretation of the results of these trials.
Evaluation of responses to NAC using magnetic resonance imaging (MRI) has been well established as a method superior to other assessment modalities, such as mammography, ultrasound, and palpation (8910). We know that dynamic contrast material-enhanced MRI most accurately assesses tumor response after NAC (911). However, it is uncertain whether patients with MRI-predicted radiologic complete response (rCR) have similar survival outcomes to patients with pCR. We also wondered if the value of MRI as a prognostic factor for NAC response would vary according to molecular subtypes, as it is well known that different breast cancer subtypes display notable differences in outcomes and responses to standard therapies (1213). To the best of our knowledge, none of the prior studies have focused on the value of response prediction by MRI as a prognostic factor for different breast cancer molecular subtypes.
Therefore, the purposes of our study were to evaluate the relationship between response categories assessed by MRI or pathology and survival outcomes following NAC, and to determine whether the prognostic value of these response categories differs among the various molecular subtypes.
Our Institutional Review Board approved this retrospective study and waived the requirement for informed consent. We reviewed data from our institution's breast surgery database between January 2007 and December 2010. Patients with biopsy-confirmed invasive breast cancer who had undergone MRI before and after NAC but before surgery were included in this study. A total of 258 patients (mean age, 44.4 years; range, 23-72 years) were recruited into this study. Patients were excluded if they had histologically confirmed distant metastasis at the time of diagnosis, had malignancy of other organs (n = 2), or had bilateral breast cancer (n = 6).
After reviewing the patients' medical records, we identified four commonly used NAC regimens: adriamycin with cyclophosphamide (AC), adriamycin with docetaxel (AT), adriamycin with cyclophosphamide plus docetaxel (AC-T), and human epidermal growth factor receptor 2 (HER2)/neu monoclonal antibody-based regimens. The HER2/neu monoclonal antibody-based regimens included AC-T with trastuzumab, docetaxel with pertuzumab, docetaxel with trastuzumab, and trastuzumab with pertuzumab. In an effort to evaluate the data under more homogeneous conditions, we also excluded the patients who were treated with any other regimens (n = 58), at an outside hospital (n = 3), or who had less than 24 months follow-up (n = 15). All patients were treated with four cycles at 3-week intervals according to their regimen protocols.
After applying the criteria, a total of 174 patients (mean age, 43.8 years; range, 23-72 years) were ultimately included in our study. The patient characteristics are summarized in Table 1.
MRI was performed using a 3T Achieva scanner (Philips Medical Systems, Best, The Netherlands) with a dedicated bilateral phase array breast coil in the prone position. The MRI examination consisted of turbo spin-echo T1- and T2-weighted sequences and a 3-dimensional dynamic contrast-enhanced (DCE) sequence. The following DCE-MRI scanning parameters were used: repetition time/echo time, 5.5 msec/2.8 msec; slice thickness, 3 mm; matrix size, 500 × 237; field of view, 300 mm; and flip angle, 12°. DCE-MRI was performed with axial imaging, with one pre-contrast and four post-contrast dynamic series. Contrast-enhanced images were acquired at 1.5, 3, 4.5, and 6 minutes after contrast injection. Image subtraction was performed after the dynamic series. For dynamic contrast enhancement, a 0.1 mmol/kg bolus of gadopentetate dimeglumine (Magnevist; Bayer Healthcare Pharmaceuticals, Montville, NJ, USA) was injected, followed by a 20 mL saline flush.
Two radiologists reviewed the images without knowledge of the pathologic outcome and reached consensus (19 and 9 years of experience, respectively, in interpreting breast MRI). To ensure interpretive consistency, MRI examinations of each patient performed both before and after NAC were reviewed in a single session. The classification of rCR was applied when all available images revealed no enhancement at the previous site of the lesion compared with normal background tissue. If subtle enhancement was noticed only in the delayed phase images and not in the early phase images, we considered it as a rCR. Patients were classified as having either a CR or a non-CR based on the MRI images. The mean interval between the first and second examination was 82 days (range, 71-200 days).
All patients underwent surgery, regardless of their response to NAC. The mean time between follow-up MRI and surgery was 9 days (range, 3-30 days).
Residual disease in the breast after NAC was categorized as: 1) no residual cancer cells; 2) ductal carcinoma in situ (DCIS) with no residual invasive cancer; or 3) residual invasive cancer. A pCR was defined as no invasive cancer; therefore, it included both categories 1) and 2) (14). Axillary lymph node status was also considered in the definition of pCR. Therefore, a pCR was defined as ypT0/is ypN0 in this study.
The expression status of the estrogen receptor (ER), progesterone receptor (PR), and HER2 was determined from histopathologic reports of core biopsies performed prior to chemotherapy (15). Samples obtained from core needle biopsy were classified as positive for ER and PR if ≥ 10% of the nuclei were stained (16). Tumors with HER2 scores of 3+ (strong homogeneous staining) were considered positive. In case of tumors with 2+ scores (moderate complete membrane staining in ≥ 10% of tumor cells), silver-enhanced in situ hybridization was used to determine HER2 amplification (gene copy number > 6 or HER2/chromosome 17 ratio > 2.2). Tumors were classified into 3 subgroups based on their receptor status in pretreatment core biopsies: triple-negative (ER-, PR-, HER2-), HER2-positive (HER2+, ER- or ER+, PR- or PR+), and ER-positive (ER+, HER2-, PR- or PR+). The other histologic features evaluated included the histologic grade, Ki-67, lymphovascular invasion, and extensive intraductal component (EIC).
Kappa statistics were used to evaluate agreement of the response categories assigned based on follow-up MRI and pathology.
The primary end point analyzed was recurrence and recurrence-free survival (RFS). Breast cancer recurrence was defined as either locoregional or distant recurrence. Locoregional recurrence was defined as recurrent disease in the ipsilateral breast or in the axillary, supraclavicular, infraclavicular, or internal mammary nodes. Recurrence at any other site was considered to be distant metastasis. We only recorded the first recurrence, and RFS was defined according to the Standardization of Events and End Points criteria (17) starting from the date of NAC initiation and ending on the date of breast cancer recurrence, date of death, date last known to have no evidence of disease, or date of the most recent follow-up.
Cox proportional hazards models were used to analyze the effect of clinicopathologic variables (age, radiologic response category, pathologic response category, clinical T stage, clinical N stage, lymphovascular invasion, histologic grade, EIC, molecular subtype, and expression status of Ki-67) on recurrence.
The Kaplan-Meier curves were used to analyze overall and molecular subtype-specific survival. Log-rank tests were used to compare differences in survival. Multivariate analyses were performed using the Cox proportional hazards model and clinicopathologic factors (clinical T stage, clinical N stage, lymphovascular invasion, molecular subtype, radiologic response category, and pathologic response category) were included in analyses.
All statistical analyses were performed using SPSS version 20.0 for Windows (IBM Corp, Armonk, NY, USA). A p value less than 0.05 was considered statistically significant.
Table 2 shows a comparison of response categories based on MRI and pathology results for each molecular subtype. Among the entire group of 174 patients, 34 patients (19.5%) showed a CR on MRI and 37 (21.3%) patients showed a pCR. The kappa value for overall agreement between radiologic and pathologic classification was 0.629, indicating that there was substantial agreement (95% confidence interval, 0.484-0.773). The kappa value was the highest in triple-negative breast cancers (kappa value = 0.778). MRI findings accurately predicted pCR in 25 patients. As shown in Table 2, rCR and pCR rates were highest in HER2-positive breast cancers (30.5% and 37.3%, respectively). ER-positive breast cancers showed the lowest rCR and pCR rates (7.5% and 3.0%, respectively).
There were 41 cases of recurrence (9 cases of locoregional recurrence and 32 cases of distant recurrence). There were no cases of in-breast recurrence. The mean time to recurrence was 19.3 months (range, 2-49 months). Disease recurrence was observed in 20 patients with triple-negative breast cancer (20/48, 41.7%), seven patients with HER2-positive breast cancer (7/59, 11.9%), and 14 patients with ER-positive breast cancer (14/67, 20.9%). The mean follow-up time was 54.8 months (range, 24-89 months).
Figure 1 shows that there was a statistically significant difference in RFS between patients who did and did not achieve a CR as assessed by both radiologic and pathologic methods (p = 0.003 and 0.013, respectively). As shown in Table 3, there were statistically significant differences in recurrence hazard ratio according to the radiologic response category, pathologic response category, and lymphovascular invasion (hazard ratio, 11.02; p = 0.018, hazard ratio, 3.93; p = 0.022, hazard ratio, 2.44; p = 0.008, respectively). When analyzed according to the molecular subtype, only patients with triple-negative breast cancer showed a significantly higher recurrence hazard ratio (hazard ratio, 2.49; p = 0.009). Kaplan-Meier curves for RFS in patients with CR versus non-CR assessed radiologically or pathologically for each molecular subtype are shown in Figure 2. By both radiologic and pathologic assessments, triple-negative breast cancer was the only subtype that showed significantly better outcomes in patients who achieved a CR compared to patients who did not achieve a CR (p = 0.004 and 0.001, respectively). Patients with ER-positive and HER2-positive breast cancer who achieved a CR did not have significantly better RFS compared to patients with a non-CR when responses were assessed either radiologically or pathologically (ER-positive, p = 0.291, 0.341, respectively; HER2-positive, p = 0.360, 0.337, respectively).
Multivariate analysis using the Cox proportional hazards model showed that, although patients who achieved a rCR or a pCR were less likely to experience recurrence than patients who did not achieve a CR, the difference was not statistically significant (hazard ratios = 2.02 and 1.12, respectively; p = 0.505 and 0.869, respectively) (Table 4). Patients with lymphovascular invasion were more likely to have recurrences, and this difference was statistically significant according to both radiologic and pathologic assessments (hazard ratio = 3.13; p = 0.003 and hazard ratio = 3.33; p = 0.002, respectively). When we examined individual molecular subtypes, patients with triple-negative breast cancers were more likely to experience recurrence compared to patients with ER-positive breast cancers. The difference was statistically significant (hazard ratio = 5.64; p < 0.001 and hazard ratio = 5.74; p < 0.001, respectively).
The clinical significance of pCR is a controversial issue which has been the topic of ongoing debate. Many researchers have reported improved long-term outcomes in patients with pCR compared to patients with residual tumor at the time of surgery (18192021). Therefore, achieving a pCR has been considered to be the ultimate goal for a favorable prognosis. Although most NAC trials have shown that pCR is associated with favorable outcomes, this phenomenon was not seen in the National Surgical Adjuvant Breast and Bowel Project B-27 neoadjuvant trial. In that trial, a significant number of patients did achieve a pCR when paclitaxel was added to a doxorubicin-based chemotherapy regimen, but the improvement in the pCR rate was not significantly associated with prolonged RFS or overall survival (22). Several recent studies also confirmed these observations (2324). Based on these findings, we questioned whether patients who achieved a pCR had favorable outcomes compared to patients without a pCR. We were also interested in determining the clinical significance of rCR, as predicted by MRI, and whether patients who achieved a rCR showed similar survival outcomes to patients with a pCR. In our univariate analysis, there were statistically significant differences in recurrence outcomes between patients with a CR versus a non-CR based on both radiologic and pathologic assessments. However on multivariate analysis, patients with a CR based on MRI and pathology did not have significant differences in recurrence outcomes.
Most published clinical trials for breast cancers have been conducted in heterogeneous groups of patients with mixed molecular disease subtypes. Esserman et al. (25) recently evaluated whether response to therapy (i.e., a pCR) would predict a RFS outcome, both overall and within biologic and imaging subsets. Their study of 221 patients showed that the rate of pCR and the significance of a pCR differed among the different receptor subsets. In their study, the rate of pCR was highest in patients with hormone receptor-negative/HER2-positive breast cancers (45%) and lowest in patients with hormone receptor-positive/HER2-negative breast cancers (9%). Additionally, a pCR was more predictive of RFS within each established receptor subset than in the overall population. More recently, Cortazar et al. (23) reported that the association between a pCR and long-term outcomes was weakest for hormone-receptor-positive and low-grade tumors, as well as for HER2-positive and hormone-receptor-positive tumors. Similarly, in studies by von Minckwitz et al. (1921), the association between a pCR and long-term outcomes was strongest in patients with aggressive breast cancer subtypes, while a pCR was not prognostic in patients with luminal A, luminal B, or HER2-positive breast cancers. Our results were consistent with these findings. In our study, there were significant differences in recurrence outcomes and overall RFS in patients with a rCR versus a non-rCR, and in patients with a pCR versus a non-pCR on univariate analyses and Kaplan-Meier curves. However, when analyzed according to molecular subtypes, triple-negative breast cancer was the only subset that showed a significantly different RFS between patients with a rCR versus a non-rCR and between patients with a pCR versus a non-pCR. It is relatively well established that pCR has a lower prognostic ability for ER-positive tumors, and our results support this idea (1925). Similar to our findings for pathologic assessment, survival outcomes did not differ significantly between ER-positive breast cancer patients with a rCR and a non-rCR. Although rCR and pCR rates were the lowest in ER-positive breast cancers, recurrence was also less frequent. Many studies have shown that hormone receptor-positive/HER2-negative breast cancers are the least sensitive to adjuvant or NAC, but they show good prognosis (122627). This demonstrates that being refractory to chemotherapy does not necessarily indicate poor prognosis in ER-positive breast cancers.
Kappa values of agreement between rCR and pCR were substantial. The definition of a pCR used in this study was ypT0/is ypN0, a definition that includes DCIS. Because DCIS and invasive cancer were not differentiated on MRI, only the invasive tumor was regarded as residual disease in our study. Therefore, in tumors combined with DCIS, overestimation on MRI is inevitable and causes low agreement between radiologic and pathologic assessments. We have reported this issue in our prior study (28).
Our study had several limitations. First, we did not evaluate the status of adjuvant hormonal therapy or adjuvant chemotherapy; this could have affected the survival outcomes. Second, this study was retrospective in nature and had a relatively short follow-up period. Also, the short follow-up might partially explain why the number of patients with recurrence was small, making it difficult to show a robust survival outcome. As patients with ER-positive breast cancers have been shown to have very low rates of early recurrence as well as low rates of response to chemotherapy, a longer follow-up is required to fully explore this issue (2629). Third, we did not analyze HER2-positive breast cancers according to hormone receptor status and the specific NAC regimen with or without a HER2-targeted drug. As a result, the HER2-positive breast cancer group was heterogeneous in our study. Fourth, the number of patients with recurrence was so small that we could not perform multivariate analyses to evaluate recurrence outcomes between patients with CR and without CR according to molecular subtypes. Fifth, we did not perform a quantitative analysis that used size or volumetric measurements.
In conclusion, patients with a rCR showed similar recurrence outcomes and RFS to patients with a pCR. CR based on MRI and pathology following NAC could be a useful prognostic factor for triple-negative breast cancer. Molecular subtypes and lymphovascular invasion are independent prognostic factors for recurrence. However, to obtain more robust evidence of survival difference according to the molecular subtype, a larger study is necessary.
Figures and Tables
 | Fig. 1Kaplan-Meier curves for survival based on magnetic resonance imaging assessment category (A) or pathologic assessment category (B).CR = complete response
|
 | Fig. 2Kaplan-Meier curves for survival in each breast cancer molecular subtype.Radiologic assessment in triple-negative breast cancer (A), HER2-positive breast cancer (B), and ER-positive breast cancer (C). Pathologic assessment in triple-negative breast cancer (D), HER2-positive breast cancer (E), and ER-positive breast cancer (F). CR = complete response, ER = estrogen receptor, HER2 = human epidermal growth factor receptor 2
|
Table 1
Clinicopathologic Features of 174 Patients Treated with Neoadjuvant Chemotherapy

Table 2
Comparisons of Response Categories Assigned Based on MRI and Pathology
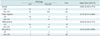
Table 3
Univariate Analysis of Recurrence

Table 4
Multivariate Analysis of Recurrence

References
1. Gralow JR, Burstein HJ, Wood W, Hortobagyi GN, Gianni L, von Minckwitz G, et al. Preoperative therapy in invasive breast cancer: pathologic assessment and systemic therapy issues in operable disease. J Clin Oncol. 2008; 26:814–819.
2. Rastogi P, Anderson SJ, Bear HD, Geyer CE, Kahlenberg MS, Robidoux A, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008; 26:778–785.
3. van der Hage JA, van de Velde CJ, Julien JP, Tubiana-Hulin M, Vandervelden C, Duchateau L. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol. 2001; 19:4224–4237.
4. Buchholz TA, Lehman CD, Harris JR, Pockaj BA, Khouri N, Hylton NF, et al. Statement of the science concerning locoregional treatments after preoperative chemotherapy for breast cancer: a National Cancer Institute conference. J Clin Oncol. 2008; 26:791–797.
5. von Minckwitz G, Blohmer JU, Costa SD, Denkert C, Eidtmann H, Eiermann W, et al. Response-guided neoadjuvant chemotherapy for breast cancer. J Clin Oncol. 2013; 31:3623–3630.
6. Cameron DA, Anderson ED, Levack P, Hawkins RA, Anderson TJ, Leonard RC, et al. Primary systemic therapy for operable breast cancer--10-year survival data after chemotherapy and hormone therapy. Br J Cancer. 1997; 76:1099–1105.
7. Liedtke C, Mazouni C, Hess KR, André F, Tordai A, Mejia JA, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008; 26:1275–1281.
8. Abraham DC, Jones RC, Jones SE, Cheek JH, Peters GN, Knox SM, et al. Evaluation of neoadjuvant chemotherapeutic response of locally advanced breast cancer by magnetic resonance imaging. Cancer. 1996; 78:91–100.
9. Balu-Maestro C, Chapellier C, Bleuse A, Chanalet I, Chauvel C, Largillier R. Imaging in evaluation of response to neoadjuvant breast cancer treatment benefits of MRI. Breast Cancer Res Treat. 2002; 72:145–152.
10. Weatherall PT, Evans GF, Metzger GJ, Saborrian MH, Leitch AM. MRI vs. histologic measurement of breast cancer following chemotherapy: comparison with x-ray mammography and palpation. J Magn Reson Imaging. 2001; 13:868–875.
11. Rieber A, Brambs HJ, Gabelmann A, Heilmann V, Kreienberg R, Kühn T. Breast MRI for monitoring response of primary breast cancer to neo-adjuvant chemotherapy. Eur Radiol. 2002; 12:1711–1719.
12. Carey LA, Dees EC, Sawyer L, Gatti L, Moore DT, Collichio F, et al. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007; 13:2329–2334.
13. Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res. 2005; 11:5678–5685.
14. Jones RL, Lakhani SR, Ring AE, Ashley S, Walsh G, Smith IE. Pathological complete response and residual DCIS following neoadjuvant chemotherapy for breast carcinoma. Br J Cancer. 2006; 94:358–362.
15. Park YJ, Youk JH, Son EJ, Gweon HM, Kim JA. Comparison of hormonal receptor and HER2 status between ultrasound-guided 14-gauge core needle biopsy and surgery in breast cancer patients. Ultrasonography. 2014; 33:206–215.
16. Regan MM, Viale G, Mastropasqua MG, Maiorano E, Golouh R, Carbone A, et al. Re-evaluating adjuvant breast cancer trials: assessing hormone receptor status by immunohistochemical versus extraction assays. J Natl Cancer Inst. 2006; 98:1571–1581.
17. Hudis CA, Barlow WE, Costantino JP, Gray RJ, Pritchard KI, Chapman JA, et al. Proposal for standardized definitions for efficacy end points in adjuvant breast cancer trials: the STEEP system. J Clin Oncol. 2007; 25:2127–2132.
18. Chaturvedi S, McLaren C, Schofield AC, Ogston KN, Sarkar TK, Hutcheon AW, et al. Patterns of local and distant disease relapse in patients with breast cancer treated with primary chemotherapy: do patients with a complete pathological response differ from those with residual tumour in the breast? Breast Cancer Res Treat. 2005; 93:151–158.
19. von Minckwitz G, Untch M, Blohmer JU, Costa SD, Eidtmann H, Fasching PA, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30:1796–1804.
20. von Minckwitz G, Untch M, Loibl S. Update on neoadjuvant/preoperative therapy of breast cancer: experiences from the German Breast Group. Curr Opin Obstet Gynecol. 2013; 25:66–73.
21. von Minckwitz G, Untch M, Nüesch E, Loibl S, Kaufmann M, Kümmel S, et al. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat. 2011; 125:145–156.
22. Bear HD, Anderson S, Smith RE, Geyer CE Jr, Mamounas EP, Fisher B, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006; 24:2019–2027.
23. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014; 384:164–172.
24. Yi A, Cho N, Im SA, Chang JM, Kim SJ, Moon HG, et al. Survival outcomes of breast cancer patients who receive neoadjuvant chemotherapy: association with dynamic contrast-enhanced MR imaging with computer-aided evaluation. Radiology. 2013; 268:662–672.
25. Esserman LJ, Berry DA, DeMichele A, Carey L, Davis SE, Buxton M, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL--CALGB 150007/150012, ACRIN 6657. J Clin Oncol. 2012; 30:3242–3249.
26. Berry DA, Cirrincione C, Henderson IC, Citron ML, Budman DR, Goldstein LJ, et al. Estrogen-receptor status and outcomes of modern chemotherapy for patients with node-positive breast cancer. JAMA. 2006; 295:1658–1667.
27. Hayes DF, Thor AD, Dressler LG, Weaver D, Edgerton S, Cowan D, et al. HER2 and response to paclitaxel in node-positive breast cancer. N Engl J Med. 2007; 357:1496–1506.
28. Ko ES, Han BK, Kim RB, Ko EY, Shin JH, Hahn SY, et al. Analysis of factors that influence the accuracy of magnetic resonance imaging for predicting response after neoadjuvant chemotherapy in locally advanced breast cancer. Ann Surg Oncol. 2013; 20:2562–2568.
29. Knauer M, Mook S, Rutgers EJ, Bender RA, Hauptmann M, van de Vijver MJ, et al. The predictive value of the 70-gene signature for adjuvant chemotherapy in early breast cancer. Breast Cancer Res Treat. 2010; 120:655–661.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download