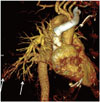
A 59-year-old man presented a six-month history of worsening dyspnea and dry cough, and not responding to medication. There was no history of fever, hematemesis, loss of appetite, or chest pain. Physical examination revealed peripheral cyanosis and clubbing. Bibasilar crepitations were present but no murmur was auscultated. Platypnea (worsening of dyspnea in a standing position) was present. Arterial gas analysis showed a PaO2 of 52 mm when breathing ambient room air. Laboratory findings were significant for a mildly abnormal liver function test. The chest radiograph revealed reticulo-nodular opacities in the bilateral upper and lower lungs. A high resolution CT of lung showed mild diffuse subpleural septal thickening with abnormally dilated vessels in the periphery of both lower lungs. Because the extent of interstitial lung disease did not explain the persistent hypoxemia, a decision to perform a CT pulmonary angiogram (CTPA) was taken to evaluate for pulmonary thromboembolism. A CTPA ruled out pulmonary embolism and in addition showed an aneurysmal dilatation of the pulmonary artery branches in the periphery of lower lobes (Fig. 1). An incidental note was made of a nodularity of the liver margins and large gastro-oesophageal varices, prompting a multiphasic contrast enhanced CT of the liver that confirmed a cirrhotic liver with stigmata of portal hypertension. An echocardiography showed normal cardiac function, with normal pressures in the pulmonary arteries. A contrast echocardiography, using agitated saline, showed delayed appearance of air bubbles in the left atrium between 4-5 heartbeats, excluding an intracardiac shunt and supporting the diagnosis of a functional hepatopulmonary shunt. The findings were compatible with hepatopulmonary syndrome (HPS), secondary to cryptogenic cirrhosis.
The HPS consists of a triad of: 1) chronic liver disease; 2) PaO2 < 70 mm Hg or alveolar-arterial oxygen gradient > 20 mm Hg; and 3) evidence of intrapulmonary vascular dilatation (1). A majority of the patients present symptoms and signs of liver disease. The underlying pathophysiology of HPS still remains unclear, ever since it was first described in the literature. The dilatation of small peripheral pulmonary vessels is the hallmark of HPS. Vasodilatation leads to severe hypoxemia, the essential component of HPS. Three mechanisms have been suggested to explain the hypoxemia because of vasodilatation; ventilation-perfusion mismatch, diffusion-perfusion limitation and intrapulmonary shunting. Chest CT shows multiple dilated vessels extending till the pleural surface (2). The ratio of the lower lobe segmental pulmonary artery branches to the adjacent bronchiole is increased and may approach 2:1 (3). A saline-agitated contrast-enhanced trans-thoracic echocardiography is a useful tool in confirming an intrapulmonary shunt and excluding an intracradiac shunt. In the presence of an intracardiac shunt, like an atrial septal defect, bubbles from the agitated saline will appear in the left heart within 3 heartbeats after its appearance in right heart chambers. In an intrapulmonary shunt, the bubbles will appear between 4 to 6 heartbeats after their appearance in the right heart.
This case highlights the usefulness of a CTPA in evaluation of suspected cases of HPS by showing an aneurysmal dilatation of peripheral branches of the pulmonary artery.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download