Abstract
Sclerosing pneumocytoma (SP) of the lung is a rare benign neoplasm. Here, we describe an unusual presentation of SP with a wax-and-wane pattern of growth in a 47-year-old woman. Tumor diameter decreased over a 3-year follow-up period and then increased on serial follow-up computed tomography scans. The mass showed high signal intensity on both T1- and T2-weighted chest magnetic resonance imaging (MRI) and early enhancement with a plateau on dynamic MRI. We speculate that intratumoral bleeding and resorption processes accounted for the changes in tumor size.
Sclerosing pneumocytoma (SP) of the lung is a rare benign pulmonary tumor that predominantly affects middle-aged women > 50 years of age (1). SP has been traditionally called sclerosing hemangioma, because it was thought to be endothelial and vascular in origin. However, more recent immunohistochemical studies have revealed that SP originates from undifferentiated respiratory epithelium, most likely type II alveolar pneumocytes (23). The size of SP varies from subcentimeter to centimeter levels (4), and the tumor is typically solitary; however, cases of multiple lesions have been reported. Some studies have reported a few cases involving an increase in lesion size, but no cases of an alternating size change, such as a decrease followed by an increase in size, have been reported.
Here, we report a rare case of SP with a wax-and-wane growth pattern, as seen on three-dimensional (3D) computed tomography (CT) volumetric data. The dynamic magnetic resonance image (MRI) findings are also described. This study was approved by the Institutional Review Board of our hospital. The requirement for patient informed consent was waived because of the retrospective nature of the study.
A 47-year-old woman presented with an abnormal chest radiograph taken during a health examination. She was a non-smoker without any respiratory complaints.
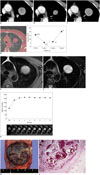
All laboratory findings were within normal limits. A round, well-circumscribed mass (approximate diameter, 3.3 cm) was observed in the left peri-hilar area on the chest radiograph. An initial contrast-enhanced chest CT scan (Fig. 1A) revealed a well-defined round mass (diameter, 3.1 cm) with heterogeneous enhancement in the left upper lobe. The mass showed a wax-and-wane growth pattern from October 2010 to October 2013 with diameters of 3.1, 2.1, 2.3, and 3.4 cm per annum, respectively (Fig. 1A-C). The volumes of the tumor measured on 3D CT using an automated segmentation technique were 15.8, 5.6, 6.7, and 20.0 cm3 per annum, respectively (Fig. 1D).
MRI was performed with a 1.5 T MR scanner (MAGNETOM Avanto, Siemens Healthcare, Erlangen, Germany). The mass was well-circumscribed in appearance and showed iso- to high signal intensity (SI) on the T1-weighted image (T1WI) (Fig. 1E) and high SI on the fat saturated T2-weighted image (T2WI) (Fig. 1F). Dynamic enhanced MRIs were obtained using a T1-weighted fat-saturated 3D gradient echo sequence (volume interpolated breath-hold gradient-echo examination [VIBE]) after administering gadolinium. The imaging parameters for the VIBE sequence were: repetition time/echo time, 4.06/1.81 ms; flip angle, 10°; slice thickness, 2.5 mm with no interslice gap; and matrix size, 384 × 202. Patients underwent a pre-contrast VIBE, followed by contrast-enhanced VIBE every 1 minute for 8 minutes. The region of interest was drawn at the largest cross-sectional diameter of the mass without violating the adjacent normal lung tissue. The lesion revealed early enhancement without a definite peak point and a subsequent plateau pattern during the dynamic MRI examination (Fig. 1G).
The tumor was resected completely during a left upper lobectomy under thoracotomy. The mass was located on the anterior segment of the left upper lobe and had a longest diameter of 3.3 cm. The cut surface of the lesion revealed a solid, well-demarcated mass with hemorrhaging (Fig. 1H). The tumor demonstrated blood-filled cystic spaces lined with cuboidal surface cells and round stromal cells on a microscopic examination (Fig. 1I). Immunohistochemical staining was positive for thyroid nuclear factor-1 (TTF-1) and the Ki-67 antigen, but negative for Napsin A and smooth muscle actin. The final pathological diagnosis was a SP.
Sclerosing pneumocytoma was first described by Liebow and Hubbell (5). These tumors are mostly found incidentally on chest radiographs, but some patients may present with chest pain, cough, and hemoptysis. Lymph node metastases have been reported in a few cases but did not seem to affect patient prognosis (6). SP is generally regarded as a benign neoplasm.
Sclerosing pneumocytoma was originally thought to be of endothelial or vascular origin because of its rich vascular structure. However, more recent ultrastructural and immunohistochemical studies have shown that SP originates from persisting primitive respiratory epithelium, including cuboidal surface cells and round cells. SP typically shows four architectural patterns histopathologically, such as solid, papillary, angiomatous, and sclerotic components. SP is diagnosed if epithelial membrane antigen and TTF-1 are expressed by both surface and round cells on an immunohistochemical evaluation (27).
Sclerosing pneumocytoma typically presents as a well-defined juxtapleural or peri-hilar mass with inhomogeneous enhancement ranging from 67-112 Hounsfield units (HU) (8) or 96-157 HU on conventional CT scans (9). SP may have variable attenuation areas within the tumor, which correspond to its histopathological features, including solid, papillary, angiomatous, and sclerotic components.
Sclerosing pneumocytoma may grow, although it is pathologically benign. However, no case of alternating size change has been reported previously. The current case showed a wax-and-wane growth pattern, with a decrease in size from 3.1 to 2.1 cm in the longest diameter, followed by an increase in size to 3.4 cm over a 3-year follow-up period. Although the reason for this observation is not completely clear, we believe that intratumoral hemorrhaging, which can be found in SP due to the bleeding potential of the angiomatous component, is a possible explanation for the wax-and-wane size change, with intratumoral bleeding and resorption causing the size changes.
Sclerosing pneumocytoma may show mixed areas of high and low SI on both T1WI and T2WI MRI, respectively and intense enhancement on post-contrast T1WI (3). The high-SI areas on T1WI correspond to the solid sclerotic components with abundant clear cells and the low-SI areas on T2WI correspond to the fibrotic or hemorrhagic components. The high-SI areas on T2WI, as well as the contrast-enhanced areas on post-contrast T1WI, reveal abundant blood cavities, which typically correspond to the angiomatous components of the tumor. A few reports have assessed the usefulness of dynamic MRI in a lung SP. Nakanishi et al. (10) reported that peak enhancement occurs 2 minutes after the administering gadolinium in a dynamic MRI study of a SP. The tumor in our case showed early enhancement without a definite peak time and a subsequent plateau pattern, which is suggestive of a benign rather than a malignant lesion (11).
In summary, the imaging findings of the current case are consistent with the well-known radiological feature of a SP but presented with an unusual wax-and-wane growth pattern, which was pathologically determined to be associated with intratumoral hemorrhaging.
Figures and Tables
Fig. 1
Sclerosing pneumocytoma with wax-and-wane growth pattern in 47-year-old woman.
A-C. Initial (A) and 1-year (B) and 3-year follow-up (C) contrast-enhanced computed tomography (CT) scans show heterogeneously enhancing mass in left upper lobe. Mass decreases in diameter from 3.1 to 2.1 cm and then marked increase to 3.4 cm on serial CT scans. D. Serial volume graph chart obtained from three-dimensional CT data using automated segmentation technique during follow-up shows wax-and-wane pattern. E, F. Mass shows iso- to higher signal intensity (SI) than that of muscle on T1-weighted magnetic resonance (MR) image (E) and heterogeneously high SI on fat-saturated T2-weighted image (F). G. Dynamic contrast-enhanced MR images and corresponding graph of SI versus time show early enhancement without peak point and subsequent plateau pattern. H. Gross findings show well-demarcated, solid mass with fibrous matrix and areas of hemorrhage. I. Well-demarcated mass with small cystic spaces filled with blood was observed on microscopic examination (hematoxylin and eosin staining, × 12.5).
References
1. Illei PB, Rosai J, Klimstra DS. Expression of thyroid transcription factor-1 and other markers in sclerosing hemangioma of the lung. Arch Pathol Lab Med. 2001; 125:1335–1339.
2. Devouassoux-Shisheboran M, Hayashi T, Linnoila RI, Koss MN, Travis WD. A clinicopathologic study of 100 cases of pulmonary sclerosing hemangioma with immunohistochemical studies: TTF-1 is expressed in both round and surface cells, suggesting an origin from primitive respiratory epithelium. Am J Surg Pathol. 2000; 24:906–916.
3. Fujiyoshi F, Ichinari N, Fukukura Y, Sasaki M, Hiraki Y, Nakajo M. Sclerosing hemangioma of the lung: MR findings and correlation with pathological features. J Comput Assist Tomogr. 1998; 22:1006–1008.
4. Kim GY, Kim J, Choi YS, Kim HJ, Ahn G, Han J. Sixteen cases of sclerosing hemangioma of the lung including unusual presentations. J Korean Med Sci. 2004; 19:352–358.
5. Liebow AA, Hubbell DS. Sclerosing hemangioma (histiocytoma, xanthoma) of the lung. Cancer. 1956; 9:53–75.
6. Miyagawa-Hayashino A, Tazelaar HD, Langel DJ, Colby TV. Pulmonary sclerosing hemangioma with lymph node metastases: report of 4 cases. Arch Pathol Lab Med. 2003; 127:321–325.
7. Kalhor N, Staerkel GA, Moran CA. So-called sclerosing hemangioma of lung: current concept. Ann Diagn Pathol. 2010; 14:60–67.
8. Cheung YC, Ng SH, Chang JW, Tan CF, Huang SF, Yu CT. Histopathological and CT features of pulmonary sclerosing haemangiomas. Clin Radiol. 2003; 58:630–635.
9. Im JG, Kim WH, Han MC, Han YM, Chung JW, Ahn JM, et al. Sclerosing hemangiomas of the lung and interlobar fissures: CT findings. J Comput Assist Tomogr. 1994; 18:34–38.
10. Nakanishi K, Kohzaki S, Fujimoto S, Horita Y, Hayashi K. Pulmonary sclerosing hemangioma: report of a case with emphasis on dynamic MR imaging findings. Radiat Med. 1997; 15:117–119.
11. Kono R, Fujimoto K, Terasaki H, Müller NL, Kato S, Sadohara J, et al. Dynamic MRI of solitary pulmonary nodules: comparison of enhancement patterns of malignant and benign small peripheral lung lesions. AJR Am J Roentgenol. 2007; 188:26–36.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download