Abstract
Pulmonary vein (PV) stenosis is a complication of ablation therapy for arrhythmias. We report two cases with chronic lung parenchymal abnormalities showing no improvement and waxing and waning features, which were initially diagnosed as nonspecific pneumonias, and finally confirmed as PV stenosis. When a patient presents for nonspecific respiratory symptoms without evidence of infection after ablation therapy and image findings show chronic and repetitive parenchymal abnormalities confined in localized portion, the possibility of PV stenosis should be considered.
Pulmonary vein (PV) stenosis is a relatively uncommon complication of radiofrequency ablation (RFA) therapy for arrhythmias, caused by thermal injury of the vessel wall (1). This condition is usually asymptomatic, and but in severe cases can lead to pulmonary venous infarction, resulting in respiratory symptoms (2). However, due to the nonspecific nature of the symptoms and imaging findings in cases of stenosis, it is easy to misdiagnose pulmonary venous infarction as parenchymal disease or infection. There have been only a few reports focusing on the radiologic findings in the pulmonary parenchyma caused by PV stenosis after ablation therapy (34). This report describes imaging findings in the lung parenchyma and sequential changes related to pulmonary venous infarction and stenosis caused by ablation therapy in two patients.
A 49-year-old man with a long-standing history of atrial fibrillation (AF) refractory to pharmacologic therapy underwent RFA at an outside hospital. Before the ablation, all four PVs were mapped by cardiac computed tomography (CT) with three-dimensional reconstruction. After successful ablation therapy, the patient took daily aspirin to prevent recurrence of AF.
Sixteen months later, the patient revisited the hospital because of a small amount of recurrent hemoptysis and left-sided chest pain. Physical examination and laboratory studies were within normal limits. Chest radiography showed a focal linear opacity with consolidation in the left upper lung zone and non-enhanced CT revealed ill-defined nodular lesions and a parenchymal band in the left upper lobe (Fig. 1A, B). A definitive diagnosis was not made at that time since the patient had no evidence of infection and CT findings did not correlate with the typical presentation of pneumonia, interstitial lung disease, or vasculitis. As a result, the patient was diagnosed with organizing pneumonia with plans for a follow-up CT study two months later.
Without improvement in symptoms, the follow-up non-enhanced CT scan revealed disappearance of the nodular lesions but new areas of lobular consolidation and thickening of the parenchymal band in the left upper lobe (Fig. 1C, D). The patient presented to our hospital with recurrent hemoptysis three months later despite discontinuing his aspirin use. CT revealed new nodular lesions and patchy consolidation in the left upper lobe (Fig. 1E, F). Although there was no significant stenosis of PV on pre-ablation cardiac CT (Fig. 1G) and sixteen months follow-up CT (Fig. 1H), severe stenosis of the left superior PV was noted on contrast-enhanced image (Fig. 1I, J). The remaining three PVs were normal in appearance. Transbronchial lung biopsy results showed interstitial septal thickening and fibrosis.
A 50-year-old woman with AF underwent RFA. Before the ablation, all four PVs were mapped by cardiac CT. During the procedure, AF originating from the PV-antrum, and ventricular premature complexes originating from the right ventricular outflow tract area were noted.
Seven months after the procedure, the patient began to experience chest discomfort. Non-enhanced CT showed ground-glass opacity nodular lesions in the left lower lobe with left pleural effusion (Fig. 2A, B). The patient was subsequently diagnosed with organizing pneumonia. However, one year later, the patient presented with hemoptysis and dyspnea. Transthoracic echocardiography showed no definite abnormality. A follow-up contrast-enhanced CT was done, which showed new nodular lesions and interstitial septal thickening in the left lower lobe (Fig. 2C, D). Comparing with pre-ablation cardiac CT (Fig. 2E), an occluded left inferior PV was newly noted (Fig. 2F). A ventilation/perfusion lung scan showed absent perfusion of the left lower lobe (Fig. 2G). Under the impression of left inferior PV stenosis with non-function of involved lung parenchyma, a left lower lobectomy was performed. Histopathologic examination showed intimal hyperplasia associated with hypertensive pulmonary vasculopathy and multifocal hemorrhagic infarction secondary to PV thrombosis.
Pulmonary vein stenosis is one of the complications associated with RFA, having once occurred in 42.4% of patients (5). With increased experience and development of new procedural techniques, the incidence has fallen to 1.3% (6). As autonomic triggers of AF originate in the roots of the PVs in about 90% of cases, specifically the left superior PV, ablation therapy isolates the PV from the left atrium using electrical energy (1). The mechanism of PV stenosis is scarring and contraction of the venous wall by thermal injury. This corresponds to histopathologic findings such as thickening of the venous wall caused by architectural remodeling and neointimal hyperplasia leading to luminal stenosis of the vein (27).
Patients may present with nonspecific respiratory symptoms, such as dyspnea on exertion, cough, chest pain, or hemoptysis (2). The severity of symptoms relates to the number of veins involved, the degree and length of luminal stenosis, and the duration of disease (4). Because the presenting symptoms are typically respiratory in nature, these patients are typically evaluated by pulmonologists instead of cardiologists. Clinicians and radiologists may make an erroneous diagnosis of bronchitis or other pulmonary parenchymal disease. Both patients in our cases were diagnosed with organizing pneumonia based on non-enhanced CT findings, delaying the establishment of PV stenosis. Although there have been many studies of PV stenosis, they have largely concentrated on the degree of venous stenosis itself and its correlation with severity of symptoms, mechanism, or treatment in the field of cardiology, and not on the consequent lung parenchymal changes (2378910).
Contrast-enhanced CT reveals pulmonary venous occlusion directly, but it is difficult to detect PV stenosis in cases of mild PV stenosis (2). Lung parenchymal abnormalities are indirect evidences of PV stenosis. As parenchymal changes are accompanied only by hemodynamically important stenosis, these are more significant findings than just narrowing of PV (3). The findings within the lung parenchyma include multifocal peripheral opacities or nodular lesions consistent with venous infarction or alveolar hemorrhage, interstitial septal thickening and mediastinal fibrosis. Interstitial septal thickenings or parenchymal bands are the result of fibrous connective tissue hyperplasia and intimal hypertrophy of venules, which are specifically seen in the chronic phase of pulmonary venous obstruction (8). In our cases, serial CT images showed repetition of appearance and disappearance of multifocal nodular lesions accompanied by persistent interstitial septal thickening in the chronic phase. So, the waxing and waning form of the chronic parenchymal abnormalities in a distribution that is limited to the involved vein can be helpful in diagnosing selective PV stenosis even on non-enhanced images.
Increased attenuation of the mediastinal fat adjacent to the site of stenosis is a finding that results from inflammation and mediastinal fibrosis due to thermal injury (3). However, when stenosis is accompanied by mediastinal findings, idiopathic mediastinal fibrosis must be excluded before a diagnosis of ablation-induced stenosis can be made (8).
Pürerfellner et al. (9) suggested a staged approach for diagnosing PV stenosis with transesophageal echocardiography in addition to CT. Echocardiography shows increases in venous flow at the site of stenosis, showing high sensitivity in the superior PVs (2). Ventilation/perfusion scans can show perfusion defects limited to the distribution of the involved vein when irreversible physiological changes due to obstruction have occurred (10).
Although balloon angioplasty of the PV is first-line therapy for this condition, the rate of restenosis is relatively high. Surgery can be considered in patients who receive repetitive angioplasty or have non-functional areas of the lung (3).
In conclusion, chronic parenchymal abnormalities showing no improvement, or that wax and wane in a localized portion of one lobe, are important clues to the diagnosis of acquired selective PV stenosis, even on non-enhanced CT. When a patient presents for respiratory symptoms with a history of RFA and imaging findings show lung parenchymal changes, one should consider the possibility of PV stenosis with sequential changes of involved lung after excluding the possibility of infection, interstitial lung disease, and thromboembolism.
Figures and Tables
Fig. 1
Serial imaging findings over 21-month period of 49-year-old man with history of ablation therapy.
A, B. On 16-month post-ablation non-enhanced CT (lung windows), multiple nodular lesions (arrowheads) and linear parenchymal band (arrows) in left upper lobe are detected. C, D. On 18-month post-ablation follow-up CT, multiple nodular lesions are seen to disappear, though new nodules (arrows) are visible in lingular division of left upper lobe. Interstitial septal thickening (arrowheads) is prominent. E, F. On 21-month post-ablation follow-up CT, nodular lesions had disappeared but multiple additional nodules (arrowheads) are noted in left upper lobe. G. Pre-ablation cardiac CT (mediastinal windows) shows normal left superior pulmonary vein (arrows). H. On 18-month follow-up non-enhanced CT image, there is no significant stenosis of left superior pulmonary vein (arrows). I, J. But on 21-month follow-up axial (I) and coronal (J) contrast-enhanced CT images show severe stenosis (arrowheads at stenosis site) of left superior pulmonary vein (arrows).
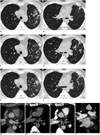
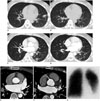
Fig. 2
Serial imaging findings over 19-month period of 50-year-old woman with history of ablation therapy.
A, B. On seven-month post-ablation non-enhanced CT (lung windows), several ground-glass opacity nodular lesions (arrow) are identified in superior segment of left lower lobe with small left pleural effusion (arrowheads). C, D. On 19-month post-ablation follow-up CT, nodular lesions and pleural effusion are seen to resolve, but new nodules in left lower lobe (arrows) are noted. Also, interstitial septal thickening in left lower lobe (black arrowhead) are newly detected. E, F. On 19-month follow-up contrast-enhanced CT images (mediastinal windows, F) show severe stenosis of left inferior pulmonary vein compared with pre-ablation cardiac CT (E, arrows at origin site of left inferior pulmonary vein). G. On ventilation/perfusion lung scan, total perfusion deficit of left lower lobe is detected.
References
1. Haïssaguerre M, Jaïs P, Shah DC, Takahashi A, Hocini M, Quiniou G, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998; 339:659–666.
2. Packer DL, Keelan P, Munger TM, Breen JF, Asirvatham S, Peterson LA, et al. Clinical presentation, investigation, and management of pulmonary vein stenosis complicating ablation for atrial fibrillation. Circulation. 2005; 111:546–554.
3. Ravenel JG, McAdams HP. Pulmonary venous infarction after radiofrequency ablation for atrial fibrillation. AJR Am J Roentgenol. 2002; 178:664–666.
4. Yataco J, Stoller JK. Pulmonary venous thrombosis and infarction complicating pulmonary venous stenosis following radiofrequency ablation. Respir Care. 2004; 49:1525–1527.
5. Chen SA, Hsieh MH, Tai CT, Tsai CF, Prakash VS, Yu WC, et al. Initiation of atrial fibrillation by ectopic beats originating from the pulmonary veins: electrophysiological characteristics, pharmacological responses, and effects of radiofrequency ablation. Circulation. 1999; 100:1879–1886.
6. Cappato R, Calkins H, Chen SA, Davies W, Iesaka Y, Kalman J, et al. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005; 111:1100–1105.
7. Ernst S, Ouyang F, Goya M, Löber F, Schneider C, Hoffmann-Riem M, et al. Total pulmonary vein occlusion as a consequence of catheter ablation for atrial fibrillation mimicking primary lung disease. J Cardiovasc Electrophysiol. 2003; 14:366–370.
8. Williamson WA, Tronic BS, Levitan N, Webb-Johnson DC, Shahian DM, Ellis FH Jr. Pulmonary venous infarction. Chest. 1992; 102:937–940.
9. Pürerfellner H, Aichinger J, Martinek M, Nesser HJ, Cihal R, Gschwendtner M, et al. Incidence, management, and outcome in significant pulmonary vein stenosis complicating ablation for atrial fibrillation. Am J Cardiol. 2004; 93:1428–1431. A10
10. Holmes DR Jr, Monahan KH, Packer D. Pulmonary vein stenosis complicating ablation for atrial fibrillation: clinical spectrum and interventional considerations. JACC Cardiovasc Interv. 2009; 2:267–276.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download