Abstract
Two angiographic instances of anomalous external carotid artery (ECA) and internal carotid artery (ICA) anastomosis are described, each occurring at the C2-3 level and bearing remnants of proximal ICA. The ICA remnant of one patient (identifiable immediately upon bifurcation of the common carotid artery) was hypoplastic, and that of the other patient was an occluded arterial stump. These features are not typical of non-bifurcating ICA. The occipital artery originated from an anomalous connection in one instance and from the main trunk of the ECA (just past the ECA-ICA connection) in the other.
The common carotid artery usually divides into the internal carotid artery (ICA) and external carotid artery (ECA) at the C3-4 level. The non-bifurcating cervical carotid artery (NBCCA) is a rare variant of the carotid arterial bifurcation, in which the ECA trunk directly arises from the common carotid artery without the actual bifurcation forming the ICA (12). Several reports have described similar anomalies showing an anomalous ECA-ICA anastomosis (12345).
However, it remains unclear how those variants arise and whether the ECA or ICA serves as the common trunk. This study encountered two patients with an anomalous ECA-ICA anastomoses, each bearing a remnant of the proximal ICA segment. These features are uncharacteristic of NBCCA. This study was approved by the Institutional Review Board of Seoul National University Hospital. The requirement to obtain written informed consent for study participation was waived.
A 44-year-old male presented with a brief loss of consciousness one month prior. He was neurologically intact at the first outpatient clinic visit. Magnetic resonance angiography (MRA) showed a suspicious cerebral aneurysm of the right A2 segment. A diagnostic cerebral angiography was performed to improve the diagnostic accuracy and to devise a therapeutic plan, and it revealed an unusual variation at the cervical segment of the carotid artery, in addition to a 5-mm right A2 aneurysm. An anomalous ECA-ICA anastomosis was identified at the C2-3 level, accompanied by a hypoplastic C4-level proximal ICA (viewed as a remnant of the normally originating ICA). The occipital artery was seen to originate from the anomalous vessel (Fig. 1). The cranial distribution of the ICA distal to the anomalous connection was otherwise normal. Coil embolization of the A2 aneurysm was planned.
A 60-year-old male was admitted to further evaluate a cerebral aneurysm incidentally discovered by an MRA during a medical check-up. Diagnostic angiography showed a right middle cerebral artery (MCA) bifurcation aneurysm, roughly 3 mm in size, with an anomalous left ECA-ICA anastomosis at the C2 level. At the C4 level, an arterial stump of the ICA was evident (Fig. 2). The occipital artery originated from the ECA main trunk, just past the anomalous connection. Surgical clipping of the right MCA bifurcation aneurysm was elected.
In these two patients, the hypoplastic proximal ICA (case 1) and the carotid arterial stump (case 2) seen on imaging, were both viewed as remnants of the proximal ICA, given that branches of the common carotid artery typically bifurcate at the C3-4 spinal level (6). The contralateral ICA of each patient originated at this level as well.
Embryologically, the ICA originated from the third aortic arch and then continues to a successive dorsal aortic arch root; and the ECA arises as a new branch from the ventral aspect of the third aortic arch (Fig. 3A-C) (12). The anomalous ECA-ICA anastomoses described here and the NBCCA seem to be similar developmental variants. There have been two hypotheses to explain the development of the NBCCA: 1) agenesis of the main trunk of the ECA and 2) agenesis of the proximal ICA segment, with persistence of the hyoid-stapedial system that connects ICA with ECA at C1 level (Fig. 3D) (124). However, demonstration of a remnant ICA or carotid stump in these two patients suggests that the NBCCA results from the normal development of the main ECA trunk along with agenesis or hypogenesis of the proximal ICA segment (Fig. 3E). In addition to the identifiable origin of ICA, the level of anomalous ECA-ICA anastomoses observed in this study (C2-3) differed from the typical level (C1), which has been reported on the NBCCA. The exact cause for discrepancy in the level of anastomoses remains elusive. However, the authors conjecture that it may be caused by downward transposition of the hyoid-stapedial system (persistence of the second aortic arch) or a development of alternate vessels other than the hyoid-stapedial system. In the second case, the carotid stump might have been caused by acquired conditions (such as dissected occlusion), in consideration of the fact that the end of the occlusion had a sharp margin.
Diminished or absent flow (of hypoplastic ICA or occluded arterial stump) may have induced the anastomoses, as in the NBCCA. Hence, the hypotheses here may simply represent variants of the NBCCA. Suzuki et al. (7) have devised a classification system for the carotid bifurcation and its branching vessels (common carotid artery, ECA, and ICA). By their criteria, patient 1 corresponds with Type C or D, whereas patient 2 (remnant carotid budding) is unclassifiable. Still, type D or E may be applied, if the arterial stump size is considered. All patterns of this classification, including these two cases and the NBCCA as well, might be understood as having the same embryological spectrum of carotid bifurcation. In terms of biologic mechanisms, the homeobox gene Hoxa-2 acts as selector gene for pathways that govern the second aortic arch structures and Hoxa-3 is required for the development of the third aortic arch artery and carotid body (89). Any defect in the regulation or expression of these genes may induce those anomalies. However, it is uncertain what defects determines the branching pattern of the carotid bifurcation.
The anomalous ECA-ICA anastomoses described here should not be confused with fenestration or duplication of the ICA. Embryologically, the proximal ICA originates from the third aortic arch, consisting of parallel, multiple vessels at an early stage (4-5 mm) (1011). If two such vessels persist instead of the usual single channel, the ICA duplication would result. Similarly, fenestration may be the consequence of an unusual carotid duct remnant, connecting third and fourth dorsal aortas (11). Instead, the anomalies in these two patients likely represent the persistence of the hyoid artery derived from the second aortic arch.
In conclusion, this study reported two instances of an anomalous ECA-ICA anastomosis with hypoplastic proximal ICA and remnant carotid budding, both confirmed by cerebral angiography. It is important to detect such developmental variants, thus avoiding arterial injuries during neck surgery or biopsy, and thereby appreciating their potential for collateral circulation during vascular compromise (arterial stenosis or occlusion).
Figures and Tables
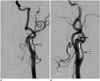 | Fig. 144-year-old man with anomalous ECA-ICA anastomosis and hypoplastic proximal ICA.
A, B. Frontal (A) and lateral (B) angiographic images showing anomalous ECA-ICA anastomosis at C2-3 spinal level and relatively hypoplastic proximal ICA at expected site of ICA origin. Anomalous anastomosis (white arrow), hypoplastic proximal ICA (arrowhead), and occipital artery (black arrow). ECA = external carotid artery, ICA = internal carotid artery
|
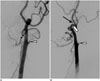 | Fig. 260-year-old man with anomalous ECA-ICA anastomosis and proximal ICA remnant.
A, B. Oblique (A) and lateral (B) angiographic images showing origins of ECA and ICA from common trunk, with carotid budding at carotid bifurcation level as probable remnant of proximal ICA. Anomalous anastomosis (white arrow), arterial stump (arrowhead), and occipital artery (black arrow). ECA = external carotid artery, ICA = internal carotid artery
|
 | Fig. 3Schematic illustration of presented case in comparison with normal ICA-ECA development and non-bifurcating cervical carotid artery.
A-C. Illustration for development of normal internal carotid artery and external carotid artery. D, E. Illustrations for typical non-bifurcating cervical carotid artery (D) and presented cases (E). a = artery, AI = first aortic arch, AII = second aortic arch, AIII = third aortic arch, AIV = forth aortic arch, CCA = common carotid artery, ECA = external carotid artery, ICA = internal carotid artery
|
References
1. Ooigawa H, Nawashiro H, Fukui S, Tsuzuki N, Katoh H, Kawaguchi T, et al. Non-bifurcating cervical carotid artery. J Clin Neurosci. 2006; 13:944–947.
2. Morimoto T, Nitta K, Kazekawa K, Hashizume K. The anomaly of a non-bifurcating cervical carotid artery. Case report. J Neurosurg. 1990; 72:130–132.
3. Kaneko K, Akita M, Murata E, Imai M, Sowa K. Unilateral anomalous left common carotid artery; a case report. Ann Anat. 1996; 178:477–480.
4. Nakai K, Kaji T, Uchino A, Kawauchi T, Tamura C, Otani N, et al. Congenital external carotid-internal carotid artery anastomosis associated with contralateral non-bifurcating cervical carotid artery. Neuroradiology. 2012; 54:521–523.
5. Rodriguez HE, Ziauddin MF, Podbielski FJ, Durham JR, Clark ET. Congenital absence of the external carotid artery: atherosclerosis without a bifurcation. J Vasc Surg. 2002; 35:573–575.
6. Klosek SK, Rungruang T. Topography of carotid bifurcation: considerations for neck examination. Surg Radiol Anat. 2008; 30:383–387.
7. Suzuki T, Moriyama T, Moriwaki H, Yagihashi A, Yajima N, Takahashi G. Anomalous artery directly connecting the external and internal carotid arteries. Ann Anat. 2000; 182:59–63.
8. Trainor PA, Krumlauf R. Hox genes, neural crest cells and branchial arch patterning. Curr Opin Cell Biol. 2001; 13:698–705.
9. Kameda Y, Watari-Goshima N, Nishimaki T, Chisaka O. Disruption of the Hoxa3 homeobox gene results in anomalies of the carotid artery system and the arterial baroreceptors. Cell Tissue Res. 2003; 311:343–352.
10. Chess MA, Barsotti JB, Chang JK, Ketonen LM, Westesson PL. Duplication of the extracranial internal carotid artery. AJNR Am J Neuroradiol. 1995; 16:1545–1547.
11. Hacein-Bey L, Raghavan N, Mukundan G, Sekhon AK, Dodrill LK, Park TC. Fenestration of the petrous internal carotid artery with short segment duplication mimicking an arterial dissection. Clin Radiol. 2013; 68:972–975.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download