Abstract
Systemic transrectal ultrasound-guided biopsy (TRUSBx) is the standard procedure for diagnosing prostate cancer (PCa), but reveals a limited accuracy for the detection of cancer. Currently, multiparametric MR imaging (mp-MRI) is increasingly regarded as a promising method to detect PCa with an excellent positive predictive value. The use of mp-MRI during a MRI-guided biopsy (MRGB) procedure improves the quality of a targeted biopsy. The aim of this article is to provide an overview about the MRGB technique for PCa detection, to review the accuracy and clinical indications of MRGB and discuss its current issues and further directions. A MRGB seems accurate and efficient for the detection of clinically significant PCa in men with previous negative TRUSBx. Moreover, it may decrease the detection of clinically insignificant cancers with fewer biopsy cores.
Prostate cancer (PCa) screening is performed with a digital rectal examination (DRE) and the serum prostate specific antigen (PSA) level. Patients with abnormal DRE findings or elevated PSA levels (> 2.5-4 ng/mL) are further evaluated with transrectal ultrasound-guided biopsy (TRUSBx) (1). Patient's treatment and prognosis are determined according to these three tests, but each test has its limitations: low specificity (36%) of PSA, low sensitivity (37%) of DRE and underestimation of Gleason score and tumor extent with TRUSBx (2, 3, 4, 5).
Recently, with the advances of prostate magnetic resonance imaging (MRI), especially with multiparametric MRI (mp-MRI), the accuracy for the localization and detection of PCa is improved. The use of prostate mp-MRI during an MRI-guided biopsy (MRGB) procedure is expected to improve the quality of a targeted biopsy. The aim of this article is to provide an overview about the MRGB technique for PCa detection, a review on the accuracy of MRGB compared with TRUSBx, to illustrate clinical indications of MRGB and discuss its current concerns and future directions.
MRI is a promising tool of growing importance in PCa evaluation, especially with the introduction of functional MRI techniques in the body oncology. The prostate MRI includes the conventional imaging (T2-weighted [T2W] and T1-weighted [T1W] imaging) and functional imaging (diffusion-weighted [DW], dynamic contrast-enhanced [DCE] and MR spectroscopic [MRS] imaging). The high-resolution T2W imaging is used by the assistance of functional imaging in PCa evaluation-mp-MRI, a combination of T2W imaging and at least one functional MRI technique. This mp-MRI provides an improved diagnostic performance for cancer detection and localization, staging, post-treatment follow-up, determination of aggressiveness, monitoring the therapeutic effect and guiding targeted biopsies (6, 7, 8). Currently, mp-MRI is most likely expected to be the best method for the evaluation of PCa, although more studies are remaining how to analyze and display this large amount of imaging data and how to optimally combine the data for the most accurate assessment of PCa. Table 1 summarizes imaging findings of PCa on mp-MRI as well as advantages and limitations of mp-MRI. Figure 1 demonstrates the typical MRI findings of PCa seen on mp-MRI.
Systemic TRUSBx (sampling 10-14 cores) continues to be the standard procedure for detecting PCa because it is simple to use and relatively cost effective. However, this systemic approach has following limitations: 1) low detection rate (27-40.3%) of PCa because up to 40% of PCa are isoechoic on ultrasound (US) and only 20-30% of hypoechoic lesions are PCa (9, 10, 11); 2) poor sampling of cancers located in the anterior, midline and apex of the prostate (12); 3) underestimation of Gleason score (34-46%) compared with the Gleason score determined in radical prostatectomy specimens (5, 13, 14); and yet 4) nonessential detection of microfocal cancer lesions (volume ≤ 0.5 cm3) that may be clinically insignificant, although this approach improves cancer detection (15). In addition, even a saturation biopsy (sampling 20-38 cores) that requires anesthesia has been reported to yield detection rates ranging from 14% to 41% and did not significantly increase the detection of clinically significant cancer (16).
On prostate MRI, the accuracy to detect PCa is various in each MR sequence (6, 8, 11): T2W imaging (50-90%), DW imaging (81-86%), DCE imaging (72-91%), and MRS imaging (82-90%). By using mp-MRI, a positive predictive value can be reached up to 90-98% (17, 18). Thus, during a MRGB procedure, the use of mp-MRI can increase the cancer detection rate, particularly the detection of clinically significant cancer. Furthermore, mp-MRI can provide additional information about tumor aggressiveness by evaluating the association between tumor Gleason score and imaging parameters.
In the clinical practice, there are three techniques of MRI guidance available for the performance of a targeted prostate biopsy (10, 14, 19): 1) cognitive targeting (physician performing TRUSBx after reviewing previous prostate MRI that shows a lesion); 2) MRI/TRUS fusion (software co-registration of stored MRI with real-time TRUS); and 3) direct MRI-guided biopsy (in-bore targeting). Table 2 summarizes the advantages and limitations of each MRGB technique.
MRI/TRUS fusion techniques use a registration or fusion software to allow a lesion defined on MRI to be shown on US during a TRUSBx procedure, with or without a tracking device (21, 23, 24, 25, 26). This technique develops a three-dimensional reconstruction of the prostate. Its advantages are that it is fast in an outpatient clinic setting and may decrease targeting errors of cognitive targeting. Its limitations include an indirect technique, the need of additional software and specialized operator training and incorrect co-registration errors.
The in-bore targeting is performed with a transrectal approach within a MRI bore (5, 27, 28, 29). The radiologists fuse a previous MRI representing a lesion with a contemporaneous MRI to confirm the biopsy needle localization. Ideally, a few targeted biopsy cores are sampled. For performing this technique, a dedicated MR biopsy device is needed (Invivo, Schwerin, Germany) (Invivo DynaCAD, Invivo DynaTrim, prostate needle guide and 150/175 mm 18-gauge automatic biopsy gun) (Fig. 2). Its advantages include an exact direct targeting, reducing the detection of clinically insignificant cancer and obtaining a few biopsy cores. Its limitations include a long procedure time, expensive costs, two MRI sessions performed, potential false-negative findings and position difficulties.
Currently, clinical indications of MRGB for detecting PCa are as following: 1) high suspicion of PCa based on an elevated PSA level and previously negative TRUSBx results (Fig. 3); 2) a rising PSA level and abnormal mp-MRI findings in biopsy-naive men; 3) active surveillance; and 4) biochemical failure after radiation therapy. In a number of studies, MRGB has been mainly performed in patients with elevating or persistently elevated PSA levels and previously negative TRUSBx results (7, 10, 24, 30). Only a few studies have reported the results of MRGB in biopsy-naive men with rising PSA level and abnormal mp-MRI finding (6, 21).
Recently, active surveillance is a management option that can be applied to patients with a presumed low-risk prostate cancer, followed by regular PSA measurement, DREs and annually repeated systemic TRUSBx. MRGB in active surveillance may give the potential for the early detection of Gleason growth pattern 4 or 5 containing cancers (31).
After a radiation therapy in PCa, the prostate gland loses its typical zonal anatomy on MRI and shows homogeneously low signal intensity on T2W imaging, which is insufficient for recurred PCa on T2W imaging (32). A recent study reported that MRGB using DW imaging is sufficient to confirm a positive diagnosis of recurred cancer (33).
There are limited data regarding MRGB yet and therefore, some issues/questions still need to be further resolved regarding the use of MRGB in men with the clinical suspicion of PCa in the daily practice. First, are there any differences between MRGB and standard TRUSBx in detecting clinically significant cancer? A recent study on the pooled analysis (34) reported that approximately 62% of the biopsy-naive population with elevated PSA levels revealed suspicious PCa findings on mp-MRI. Of these, about 66% of men had PCa on their biopsy including combined targeted and standard cores, while a standard biopsy only approach showed 50% detection rate for PCa. Haffner et al. (35) investigated clinically significant cancer, defined as any cancer core length greater than 5 mm or any Gleason pattern greater than 3. In their study, the targeted approach showed 98% detection accuracy for clinically significant cancers, while the standard approach showed 88% detection accuracy for clinically significant cancers. Missed cases of clinically significant cancer between targeted and standard biopsy approach were similar (13 of targeted biopsy and 12 of standard biopsy). Not any clinically insignificant cancer was detected in a man with the targeted approach and in 10% of men with the standard approach. In the population with previous negative TRUSBx results, the detection rate of PCa on MRGB was 37-59% (10, 30), while that on TRUSBx was 10-17% (36, 37, 38). For the detection of clinically significant cancer, MRGB and TRUSBx demonstrated a rate of 38-93% respectively 14-41%, respectively (7, 10, 25, 30, 39).
Second, what is the probability of the missed cancer in men with normal MRI findings? A recent study (34) reported that in a biopsy-naive population with elevated PSA levels, 38% of men had normal MRI findings and of these, about 23% demonstrated PCa on subsequent TRUSBx. However, interestingly, only 2.3% of the population with the missed cancer had clinically significant cancer that was defined as a cancer core length greater than 5 mm or any Gleason score higher than 3. Other studies reported approximately 15% men with normal MRI findings had any cancers on subsequent biopsies, but these studies did not evaluate the clinically significant cancer (27, 40, 41, 42). With those scant data, further studies with larger population are needed to validate the clinical significance of the missed cancers representing with normal findings on mp-MRI.
Third, is there a difference in the detection accuracy of all cancers between standard TRUSBx and MRGB? Such difference in all cancer detections and between the two methods can be compared on either per-patient or per-core basis (34). On a per-patient basis, the cancer detection was 36% in TRUSBx, while that of MRGB was 48%. On a per-core basis, the cancer detection was 30% in MRGB and 7% in TRUSBx, respectively.
Fourth, what is the definition of clinically significant cancer? No consistent definition of clinically significant cancer has been shown in several published studies (5, 21, 30, 35): more than 5 mm cancer core length or any Gleason score higher than 3; Gleason score of at least 7; at least 3 mm cancer core length or any Gleason score of at least 3; Gleason score higher than 4, stage more than T3a/N1 and tumor volume greater than 0.5 mL. Based on variable definitions of clinically significant cancer, the proportion of cancers detected was 19-93% using MRGB if compared with TRUSBx (10-14%) (27, 28, 34, 35, 40).
In terms of future research directions, first, comparative studies should be performed among in-bore targeting, MRI/TRUS fusion or cognitive targeting technique for the detection of PCa in men with the suspicion of PCa. More recently, a study compared cognitive targeting with MRI/TRUS fusion techniques for the detection accuracy of PCa and its results revealed no statistical difference between them (21). In particular, the in-bore targeting technique should be compared with MRI/TRUS fusion or cognitive targeting techniques for the detection of PCa because the in-bore targeting technique has several benefits such as exact direct targeting, few biopsy cores and an improved detection of clinically significant cancer, if compared with MRI/TRUS fusion or cognitive techniques. Second, a standardized definition of clinically significant cancer in PCa should be suggested, which may strengthen the true value of MRGB in the clinical practice. Third, a standardized reporting system of mp-MRI findings, such as the Prostate Imaging Reporting and Data System score recommended by the European Society of Urogenital Radiology (7), should be assessed for PCa detection and localization during MRGB. Fourth, few studies have demonstrated the utility of MRGB in patients with active surveillance and biochemical recurrence following radiotherapy. Fifth, MRGB has the potential to predict the accurate tumor Gleason score as compared with TRUSBx, which may result in a significant improvement of the pretreatment risk stratification (5). Finally, a few studies have reported the utility of TRUSBx using contrast-enhanced US or elastography for the detection of PCa (43, 44, 45). Further studies are needed to compare MRGB with standard TRUSBx using contrastenhanced US or elastography.
MRI-guided biopsy is an accurate and efficient procedure for the detection of clinically significant prostate cancer in patients with previous negative TRUSBx results. About one third of patients with normal MRI findings may avoid prostate biopsy. Moreover, it may decrease the detection of clinically insignificant cancers with fewer biopsy cores. As there are scant data yet, more comprehensive clinical research will be needed before MRGB can be widely used in the daily clinical practice.
Figures and Tables
Fig. 1
56-year-old man with stage T3a Gleason 4 + 3 prostate cancer (prostate specific antigen = 28.5 ng/mL) in left peripheral zone (PZ).
A. On axial T2-weighted image, focal mass of low signal intensity (arrow) is seen in left PZ on mid-gland level with indistinct and irregular capsular margin suggesting extracapsular extension. B, C. Mass (arrow) in left PZ shows focally high signal intensity on axial diffusion-weighted (B) and reduced apparent diffusion coefficient (ADC) value on ADC map (C) images. D. On axial color-coded ktrans map of dynamic contrast-enhanced image, mass (arrow) reveals focal, asymmetric enhancement in corresponding site of Figure 1A.

Fig. 2
In-bore targeting devices.
A. 150/175 mm 18-gauge automatic biopsy gun, prostate needle guide, bottles for sampled cores and lidocaine jelly are shown. B. Biopsy device with base plate for patient positioning (Invivo, Schwerin, Germany).

Fig. 3
74-year-old man with stage T2a Gleason score 3 + 4 prostate cancer (prostate specific antigen = 14.56 ng/mL) in right transition zone (TZ) with history of one previous negative transrectal ultrasound-guided biopsy underwent subsequent multiparametric MRI for clinical suspicion of prostate cancer.
A-D. Axial color-coded wash-in/wash-out image (A) and dynamic contrast-enhanced (D) images show asymmetric increased enhancement (cross) in right TZ. This mass demonstrates focal low signal intensity (cross) on axial apparent diffusion coefficient map (B) and T2-weighted (C) images. E. Oblique axial T2-weighted image confirms needle position (arrow) in right TZ. In this MRI-guided biopsy specimen, Gleason score 3 + 4 prostate cancer was found.
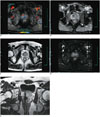
Table 1
Imaging Findings of Prostate Cancer, and Advantages and Limitations of Multiparametric MR Imaging

Table 2
Advantages, Limitations and Cancer Detection Rate of Each MR-Guided Targeted Biopsy Technique

| Targeting Technique | Advantages | Limitations | Cancer Detection | References |
|---|---|---|---|---|
| Cognitive | Simple, fast, no additional device and specialized training | Targeting errors | Overall detection rate: 54-69%, % CSC: 67-82% | Puech et al. (21) |
| Haffner et al. (35) | ||||
| MRI/TRUS fusion | Fast, ↓errors of cognitive targeting | Indirect, addition fusion or registration software, specialized training | Overall detection rate: 54-55%, % CSC: 38-73% | Rastinehad et al. (23) |
| Sonn et al. (25) | ||||
| Pinto et al. (26) | ||||
| Testa et al. (46) | ||||
| In-bore | Few cores, exact direct targeting, ↓clinically insignificant cancer detection | Time, costs, two MR sessions, false-negative findings, position difficulty | Overall detection rate: 39-59%, % CSC: 48-93% | Hambrock et al. (27) |
| Hoeks et al. (28) | ||||
| Franiel et al. (30) |
References
1. Schröder FH, Roobol MJ. Defining the optimal prostatespecific antigen threshold for the diagnosis of prostate cancer. Curr Opin Urol. 2009; 19:227–231.
2. Schröder FH, Carter HB, Wolters T, van den Bergh RC, Gosselaar C, Bangma CH, et al. Early detection of prostate cancer in 2007. Part 1: PSA and PSA kinetics. Eur Urol. 2008; 53:468–447.
3. Schröder FH, van der Maas P, Beemsterboer P, Kruger AB, Hoedemaeker R, Rietbergen J, et al. Evaluation of the digital rectal examination as a screening test for prostate cancer. Rotterdam section of the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 1998; 90:1817–1182.
4. Noguchi M, Stamey TA, McNeal JE, Yemoto CM. Relationship between systematic biopsies and histological features of 222 radical prostatectomy specimens: lack of prediction of tumor significance for men with nonpalpable prostate cancer. J Urol. 2001; 166:104–109. discussion 109-110.
5. Hambrock T, Hoeks C, Hulsbergen-van de Kaa C, Scheenen T, Fütterer J, Bouwense S, et al. Prospective assessment of prostate cancer aggressiveness using 3-T diffusion-weighted magnetic resonance imaging-guided biopsies versus a systematic 10-core transrectal ultrasound prostate biopsy cohort. Eur Urol. 2012; 61:177–184.
6. Hoeks CM, Barentsz JO, Hambrock T, Yakar D, Somford DM, Heijmink SW, et al. Prostate cancer: multiparametric MR imaging for detection, localization, and staging. Radiology. 2011; 261:46–66.
7. Barentsz JO, Richenberg J, Clements R, Choyke P, Verma S, Villeirs G, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012; 22:746–757.
8. Kim CK, Park BK. Update of prostate magnetic resonance imaging at 3 T. J Comput Assist Tomogr. 2008; 32:163–172.
9. Presti JC Jr, O'Dowd GJ, Miller MC, Mattu R, Veltri RW. Extended peripheral zone biopsy schemes increase cancer detection rates and minimize variance in prostate specific antigen and age related cancer rates: results of a community multi-practice study. J Urol. 2003; 169:125–129.
10. Eskew LA, Bare RL, McCullough DL. Systematic 5 region prostate biopsy is superior to sextant method for diagnosing carcinoma of the prostate. J Urol. 1997; 157:199–202. discussion 202-203.
11. Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT. Imaging prostate cancer: a multidisciplinary perspective. Radiology. 2007; 243:28–53.
12. Ouzzane A, Puech P, Lemaitre L, Leroy X, Nevoux P, Betrouni N, et al. Combined multiparametric MRI and targeted biopsies improve anterior prostate cancer detection, staging, and grading. Urology. 2011; 78:1356–1362.
13. Divrik RT, Eroglu A, Sahin A, Zorlu F, Ozen H. Increasing the number of biopsies increases the concordance of Gleason scores of needle biopsies and prostatectomy specimens. Urol Oncol. 2007; 25:376–382.
14. Kvåle R, Møller B, Wahlqvist R, Fosså SD, Berner A, Busch C, et al. Concordance between Gleason scores of needle biopsies and radical prostatectomy specimens: a population-based study. BJU Int. 2009; 103:1647–1654.
15. Wolters T, Roobol MJ, van Leeuwen PJ, van den Bergh RC, Hoedemaeker RF, van Leenders GJ, et al. A critical analysis of the tumor volume threshold for clinically insignificant prostate cancer using a data set of a randomized screening trial. J Urol. 2011; 185:121–125.
16. Scattoni V, Zlotta A, Montironi R, Schulman C, Rigatti P, Montorsi F. Extended and saturation prostatic biopsy in the diagnosis and characterisation of prostate cancer: a critical analysis of the literature. Eur Urol. 2007; 52:1309–1322.
17. Turkbey B, Mani H, Shah V, Rastinehad AR, Bernardo M, Pohida T, et al. Multiparametric 3T prostate magnetic resonance imaging to detect cancer: histopathological correlation using prostatectomy specimens processed in customized magnetic resonance imaging based molds. J Urol. 2011; 186:1818–1824.
18. Rouse P, Shaw G, Ahmed HU, Freeman A, Allen C, Emberton M. Multi-parametric magnetic resonance imaging to rule-in and rule-out clinically important prostate cancer in men at risk: a cohort study. Urol Int. 2011; 87:49–53.
19. Marks L, Young S, Natarajan S. MRI-ultrasound fusion for guidance of targeted prostate biopsy. Curr Opin Urol. 2013; 23:43–50.
20. Park BK, Park JW, Park SY, Kim CK, Lee HM, Jeon SS, et al. Prospective evaluation of 3-T MRI performed before initial transrectal ultrasound-guided prostate biopsy in patients with high prostate-specific antigen and no previous biopsy. AJR Am J Roentgenol. 2011; 197:W876–W881.
21. Puech P, Rouvière O, Renard-Penna R, Villers A, Devos P, Colombel M, et al. Prostate cancer diagnosis: multiparametric MR-targeted biopsy with cognitive and transrectal USMR fusion guidance versus systematic biopsy--prospective multicenter study. Radiology. 2013; 268:461–469.
22. Kumar R, Nayyar R, Kumar V, Gupta NP, Hemal AK, Jagannathan NR, et al. Potential of magnetic resonance spectroscopic imaging in predicting absence of prostate cancer in men with serum prostate-specific antigen between 4 and 10 ng/ml: a follow-up study. Urology. 2008; 72:859–863.
23. Rastinehad AR, Baccala AA Jr, Chung PH, Proano JM, Kruecker J, Xu S, et al. D'Amico risk stratification correlates with degree of suspicion of prostate cancer on multiparametric magnetic resonance imaging. J Urol. 2011; 185:815–820.
24. Natarajan S, Marks LS, Margolis DJ, Huang J, Macairan ML, Lieu P, et al. Clinical application of a 3D ultrasound-guided prostate biopsy system. Urol Oncol. 2011; 29:334–342.
25. Sonn GA, Natarajan S, Margolis DJ, MacAiran M, Lieu P, Huang J, et al. Targeted biopsy in the detection of prostate cancer using an office based magnetic resonance ultrasound fusion device. J Urol. 2013; 189:86–91.
26. Pinto PA, Chung PH, Rastinehad AR, Baccala AA Jr, Kruecker J, Benjamin CJ, et al. Magnetic resonance imaging/ultrasound fusion guided prostate biopsy improves cancer detection following transrectal ultrasound biopsy and correlates with multiparametric magnetic resonance imaging. J Urol. 2011; 186:1281–1285.
27. Hambrock T, Somford DM, Hoeks C, Bouwense SA, Huisman H, Yakar D, et al. Magnetic resonance imaging guided prostate biopsy in men with repeat negative biopsies and increased prostate specific antigen. J Urol. 2010; 183:520–527.
28. Hoeks CM, Schouten MG, Bomers JG, Hoogendoorn SP, Hulsbergen-van de, Hambrock T, et al. Three-Tesla magnetic resonance-guided prostate biopsy in men with increased prostate-specific antigen and repeated, negative, random, systematic, transrectal ultrasound biopsies: detection of clinically significant prostate cancers. Eur Urol. 2012; 62:902–909.
29. Pondman KM, Fütterer JJ, ten Haken B, Schultze Kool LJ, Witjes JA, Hambrock T, et al. MR-guided biopsy of the prostate: an overview of techniques and a systematic review. Eur Urol. 2008; 54:517–527.
30. Franiel T, Stephan C, Erbersdobler A, Dietz E, Maxeiner A, Hell N, et al. Areas suspicious for prostate cancer: MR-guided biopsy in patients with at least one transrectal US-guided biopsy with a negative finding--multiparametric MR imaging for detection and biopsy planning. Radiology. 2011; 259:162–172.
31. Somford DM, Hoeks CM, Hulsbergen-van de Kaa CA, Hambrock T, Fütterer JJ, Witjes JA, et al. Evaluation of diffusion-weighted MR imaging at inclusion in an active surveillance protocol for low-risk prostate cancer. Invest Radiol. 2013; 48:152–157.
32. Vargas HA, Wassberg C, Akin O, Hricak H. MR imaging of treated prostate cancer. Radiology. 2012; 262:26–42.
33. Rud E, Baco E, Lien D, Klotz D, Eggesbø HB. Detection of radiorecurrent prostate cancer using diffusion-weighted imaging and targeted biopsies. AJR Am J Roentgenol. 2014; 202:W241–W246.
34. Moore CM, Robertson NL, Arsanious N, Middleton T, Villers A, Klotz L, et al. Image-guided prostate biopsy using magnetic resonance imaging-derived targets: a systematic review. Eur Urol. 2013; 63:125–140.
35. Haffner J, Lemaitre L, Puech P, Haber GP, Leroy X, Jones JS, et al. Role of magnetic resonance imaging before initial biopsy: comparison of magnetic resonance imaging-targeted and systematic biopsy for significant prostate cancer detection. BJU Int. 2011; 108(8 Pt 2):E171–E178.
36. Mian BM, Naya Y, Okihara K, Vakar-Lopez F, Troncoso P, Babaian RJ. Predictors of cancer in repeat extended multisite prostate biopsy in men with previous negative extended multisite biopsy. Urology. 2002; 60:836–840.
37. Philip J, Hanchanale V, Foster CS, Javlé P. Importance of peripheral biopsies in maximising the detection of early prostate cancer in repeat 12-core biopsy protocols. BJU Int. 2006; 98:559–562.
38. Roehl KA, Antenor JA, Catalona WJ. Serial biopsy results in prostate cancer screening study. J Urol. 2002; 167:2435–2439.
39. Roethke M, Anastasiadis AG, Lichy M, Werner M, Wagner P, Kruck S, et al. MRI-guided prostate biopsy detects clinically significant cancer: analysis of a cohort of 100 patients after previous negative TRUS biopsy. World J Urol. 2012; 30:213–218.
40. Sciarra A, Panebianco V, Ciccariello M, Salciccia S, Cattarino S, Lisi D, et al. Value of magnetic resonance spectroscopy imaging and dynamic contrast-enhanced imaging for detecting prostate cancer foci in men with prior negative biopsy. Clin Cancer Res. 2010; 16:1875–1883.
41. Prando A, Kurhanewicz J, Borges AP, Oliveira EM Jr, Figueiredo E. Prostatic biopsy directed with endorectal MR spectroscopic imaging findings in patients with elevated prostate specific antigen levels and prior negative biopsy findings: early experience. Radiology. 2005; 236:903–910.
42. Lee SH, Chung MS, Kim JH, Oh YT, Rha KH, Chung BH. Magnetic resonance imaging targeted biopsy in men with previously negative prostate biopsy results. J Endourol. 2012; 26:787–791.
43. Brock M, Eggert T, Palisaar RJ, Roghmann F, Braun K, Löppenberg B, et al. Multiparametric ultrasound of the prostate: adding contrast enhanced ultrasound to real-time elastography to detect histopathologically confirmed cancer. J Urol. 2013; 189:93–98.
44. Salomon G, Köllerman J, Thederan I, Chun FK, Budäus L, Schlomm T, et al. Evaluation of prostate cancer detection with ultrasound real-time elastography: a comparison with step section pathological analysis after radical prostatectomy. Eur Urol. 2008; 54:1354–1362.
45. Woo S, Kim SY, Cho JY, Kim SH. Shear wave elastography for detection of prostate cancer: a preliminary study. Korean J Radiol. 2014; 15:346–355.
46. Testa C, Schiavina R, Lodi R, Salizzoni E, Tonon C, D'Errico A, et al. Accuracy of MRI/MRSI-based transrectal ultrasound biopsy in peripheral and transition zones of the prostate gland in patients with prior negative biopsy. NMR Biomed. 2010; 23:1017–1026.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download