Abstract
Malignant kidney neoplasms are the most frequently encountered solid kidney masses. Although renal cell carcinoma is the major renal malignancy, other solid malignant renal masses should be considered in the differential diagnosis of solid renal masses that do not contain a macroscopic fatty component. In this pictorial essay, we present the imaging findings of a primitive neuroectodermal tumor, primary liposarcoma of the kidney, primary neuroendocrine tumor, leiomyosarcoma, synovial sarcoma, malignant fibrous histiocytoma, sclerosing fibrosarcoma and renal metastasis of osteosarcoma.
The most common malignant renal tumors are renal cell carcinomas (RCC). However, unusual types of renal tumors may occur in the renal parenchyma and mimic RCC on cross-sectional imaging studies. Awareness of the imaging findings of these tumors helps radiologists with diagnosis and treatment. In this pictorial essay, we present the computed tomography (CT) and magnetic resonance imaging (MRI) features of unusual solid renal tumors, such as a primitive neuroectodermal tumor (PNET), primary liposarcoma, primary malignant neuroendocrine tumor, leiomyosarcoma (LMS), synovial sarcoma, malignant fibrous histiocytoma (MFH), sclerosing fibrosarcoma, and metastatic osteosarcoma.
A PNET is a malignant small round cell tumor characterized by aggressive biological behavior with frequent recurrence and metastatic potential (1). These tumors are most frequently encountered in adolescents and young adults. The imaging features of a PNET are indistinguishable from RCC. PNET of the kidney is mostly reported as a large mass with near-total replacement of the renal parenchyma (Fig. 1) (2). These renal lesions appear as a heterogeneous mass on CT. MRI reveals a low signal-intensity solid mass with lobulated contours on T1-weighted images and heterogeneous signal intensity on T2-weighted images. PNET of the kidney may extend to the renal veins and inferior vena cava (3).
Sarcomas of the genitourinary tract are most commonly LMSs and can be detected in prostate, urinary bladder, kidney, and paratesticular areas (45). Liposarcomas account for 20% of all soft-tissue sarcomas in adults, and they are most commonly detected at 50-65 years of age with male predominance (6). Primary renal capsule malignancies are uncommon. Primary liposarcoma of the renal capsule is an extremely rare disease with only about 20 cases reported (6).
Liposarcomas are classified into five histological subtypes, including well-differentiated, dedifferentiated, myxoid, round cell, and pleomorphic. The histological grade is the most important prognostic factor (7). The most important clinical entity in the differential diagnosis is macroscopic fat-containing angiomyolipoma. However, it may be difficult to make a confident imaging diagnosis of any kind of lipomatous tumor when the lesion does not contain macroscopic fat, as in the case presented in Figure 2.
Primary surgical resection of the mass is the only potentially curative approach. It is difficult to provide any reliable information on the long-term results of this rare tumor, given the small number of patients.
Neuroendocrine carcinomas are found in different tissues and organs, including those that do not normally contain neuroendocrine cells (8). Neuroendocrine tumors of the kidney are extremely rare neoplasms and include carcinoids, atypical carcinoids, and small cell carcinomas (9). The prognosis differs according to disease stage. Another interesting point in patients with neuroendocrine tumors is the high incidence of a horseshoe kidney (10).
The peak incidence of neuroendocrine tumors occurs in patients in their 50s and 60s but patients as young as 12 years and those in their 70s have also been reported. Older age at the time of diagnosis (> 40 years) is correlated with an advanced disease stage and a worse prognosis (9). Surgical resection is the only known curative treatment, and the prognosis may be good even in patients with local lymph node involvement (10). Metastatic disease to the liver constitutes a grave finding; however, due to the rarity of the disease, no standard treatment protocol has gained universal acceptance (11).
Among the most common radiological features of all neuroendocrine tumors is calcification (Fig. 3) (10). Although lesions may be heterogeneous on ultrasound (US) and CT, carcinoids of the kidney have a characteristic tendency to show minimal or poor enhancement on contrast-enhanced CT, corresponding to hypovascular or avascular lesions on renal angiography. Octreotide scanning has been recently proposed as a sensitive imaging modality for diagnosing and evaluating the extent of disease (10).
Renal LMS accounts for only 0.1% of all invasive renal tumors and 50-60% of all cases of renal sarcoma (12). These tumors most frequently arise from the renal capsule, smooth muscle fibers of the renal pelvis, sphincter ring around the renal papilla, and internal blood vessels (13). Spontaneous rupture of the kidney has been reported in patients with renal LMS (14). Renal LMSs appear as expansile, heterogeneously-enhancing, and well-circumscribed solid masses that usually project exophytically from the kidney (131516). Not surprisingly, the fibrous stroma of the tumor accounts for the T1- and T2-hypointensity and demonstrates delayed enhancement after intravenous injection of contrast agent (Fig. 4) (13). Large renal LMS may present as multilocular cystic masses with peripheral enhancement. A histopathological diagnosis is necessary, as these tumors cannot be distinguished from RCC and other renal malignancies.
Primary synovial sarcoma of the kidney is an extremely rare tumor. They tend to be large masses with a mean size of 8 cm (17). Renal synovial sarcomas manifest as a hypoechoic mass arising from the kidney on US. Synovial sarcoma of the kidney presents as a soft tissue mass with multiple cystic areas (Fig. 5) (3). They are usually well-demarcated, and cross-sectional imaging studies demonstrate an oval shaped, well-defined heterogeneously enhancing mass (1718). Small synovial sarcomas of the kidney usually have homogeneous signal intensity, similar to that of skeletal muscle on T1-weighted images. Synovial sarcomas > 5 cm can show heterogeneous signal intensity due to intralesional hemorrhage and necrosis (Fig. 5) (19). The cystic components of synovial sarcoma often have smooth walls unlike cystic RCCs, which usually have a mural nodule. Subcapsular hematoma secondary to tumor rupture may occur in patients with synovial sarcoma of the kidney (18).
Malignant fibrous histiocytoma of the kidney is a rare tumor, and < 100 cases have been reported. MFH arises from the kidney capsule or renal parenchyma and represents 6% of renal sarcomas (2021). Most MFHs manifest as bulky tumors with extra-renal extension (22). They tend to recur locally and can be detected in 51% of cases during the disease course (2023).
Malignant fibrous histiocytoma of the kidney presents as a well-defined soft tissue mass with inhomogeneous minimal enhancement with slightly higher attenuation than the skeletal muscle on contrast-enhanced CT (Fig. 6) (24). Decreased attenuation corresponding to regions of myxomatous tissue, cystic degeneration, hemorrhage, or necrosis is observed on CT.
Malignant fibrous histiocytomas of the kidney usually appear hypointense to kidney parenchyma on T1-weighted images, whereas they appear iso- or hyperintense on T2-weighted images (24). Hypointense areas on T1- and T2-weighted images reflect the fibrous components of the tumor and are a potentially useful clue for an imaging diagnosis of the lesion. Gradual and heterogeneous enhancement may be seen after intravenous gadolinium administration, reflecting the tumor's fibrous content (1624). Other sarcomas, sarcomatoid RCC, and malakoplakia should be considered in the differential diagnosis of renal MFH (2526).
Sclerosing fibrosarcoma of the kidney is a very rare subtype of renal sarcoma. Fibrosarcoma of the kidney usually arises from fibrous and connective tissue of the renal capsule (27). Fibrosarcomas appear as large heterogeneously enhancing soft-tissue masses on CT and MRI (Fig. 7) (13). The prognosis of this tumor is very poor, as the tumor presents with a rapid growth rate and aggressive behavior and the only potentially curative treatment is surgery (13).
Metastatic disease of the kidney is not common and is mostly seen in patients with a neoplasm of the lung, breast, gastrointestinal tract, or melanoma (28). Osteosarcoma of the kidney may be primary or more commonly metastatic. It is often bilateral, as in our case, and clinically silent (29). These lesions may show extensive calcification on radiographs, occasionally in a sunburst pattern, and may even be detectable on plain radiography (Fig. 8). Nuclear medicine scans with technetium-99 may demonstrate diffuse uptake (2). Clinically occult metastatic renal osteosarcoma is not rare and can be seen in 10% of autopsies of patients who die from osteosarcoma (30).
Solid renal masses without macroscopic fat on imaging studies are usually presumed to represent RCC. However, several malignant neoplasms may arise from the kidney. Familiarity with the imaging findings of these unusual malignant renal tumors is helpful to the practicing radiologist for a better diagnosis of these lesions.
Image-guided percutaneous renal biopsy has been increasingly used to characterize solid renal masses and may prevent unnecessary surgical intervention (31). Any lesion in the kidney can now be safely sampled with increased experience in percutaneous biopsy techniques. Percutaneous tract seeding has been a relatively common complication during biopsy of renal malignancies; however, several recent studies have demonstrated that percutaneous biopsy can be safely performed in these patients without any significant risk of tract seeding (313233).
Figures and Tables
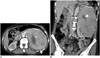 | Fig. 1Primitive neuroectodermal tumor of kidney.Axial (A) and coronal (B) post-contrast computed tomography images demonstrate large masses (arrows) arising from left kidney. Kidney parenchyma is almost completely replaced by mass. Central hypodense area (arrowheads) represents necrosis.
|
 | Fig. 2Liposarcoma of kidney.Axial post-contrast computed tomography image of 67-year-old male with surgically proven liposarcoma of kidney shows non-specific contrast enhancement and well circumscribed mass in upper pole of left kidney (arrow) with no associated macroscopic fat.
|
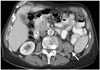
 | Fig. 3Malignant neuroendocrine tumor of kidney.Axial post-contrast computed tomography image of 52-year-old male demonstrates contrast-enhanced lesion of left kidney (arrows) that was malignant neuroendocrine tumor. Punctate focus of calcification at medial edge of lesion (arrowhead) is renal stone in adjacent calyx.
|
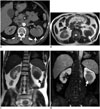 | Fig. 4Renal leiomyosarcoma.
A. Axial post-contrast computed tomography image demonstrates hypoenhancing solid mass with well-defined lobulated margins (arrow). Axial (B) and coronal (C) T2-weighted magnetic resonance image (MRI) reveals low signal intense left renal mass (arrows). D. Renal mass (arrow) appears with low enhancement on contrast-enhanced fat saturated T1-weighted MRI.
|
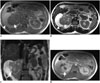 | Fig. 5Renal synovial sarcoma.Axial T1- (A) and T2-weighted (B) magnetic resonance image (MRI) demonstrate right renal mass with low signal-intensity and solid (arrows) and high signal-intensity cystic (arrowheads) components. High signal intensity in cystic portion of mass represents hemorrhagic or protein rich fluid. C. Coronal T2-weighted MRI reveals right renal mass (arrow) with peripheral cystic portion. D. Axial fat-saturated T1-weighted MRI demonstrates enhancement of solid portion (arrow) of renal synovial sarcoma.
|
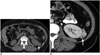 | Fig. 6Malignant fibrous histiocytoma of kidney.
A. Unenhanced axial computed tomography (CT) image demonstrates well-defined solid mass (arrow) arising from left renal capsule. B. Contrast-enhanced axial CT image reveals heterogeneous enhancement in mass (arrow).
|
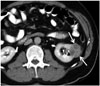
 | Fig. 7Sclerosing fibrosarcoma of kidney.Axial (A) and coronal (B) contrast-enhanced computed tomography scans show low-attenuated well-defined mass (arrow) arising from left kidney.
|
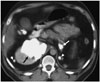 | Fig. 8Metastatic osteosarcoma in kidney.Axial post-contrast computed tomography image of 19-year-old boy with metastatic osteosarcoma of lower extremity demonstrates large calcified metastatic deposit in right kidney (black arrow). Note calcified left adrenal (white arrow) and bilateral perinephric space (arrowheads) metastases.
|
References
1. Lee H, Cho JY, Kim SH, Jung DC, Kim JK, Choi HJ. Imaging findings of primitive neuroectodermal tumors of the kidney. J Comput Assist Tomogr. 2009; 33:882–886.
2. Pickhardt PJ, Lonergan GJ, Davis CJ Jr, Kashitani N, Wagner BJ. From the archives of the AFIP. Infiltrative renal lesions: radiologic-pathologic correlation. Armed Forces Institute of Pathology. Radiographics. 2000; 20:215–243.
3. Chinnaa S, Das CJ, Sharma S, Singh P, Seth A, Purkait S, et al. Peripheral primitive neuroectodermal tumor of the kidney presenting with pulmonary tumor embolism: a case report. World J Radiol. 2014; 6:846–849.
4. Dotan ZA, Tal R, Golijanin D, Snyder ME, Antonescu C, Brennan MF, et al. Adult genitourinary sarcoma: the 25-year Memorial Sloan-Kettering experience. J Urol. 2006; 176:2033–2038. discussion 2038-2039
5. Novick AC, Campbell SC. Renal tumors. In : Walsh PC, Retik AB, Vaughan ED, Wein AJ, editors. Campbell's urology. 8th ed. Philadelphia: Saunders;2002. p. 2673–2731.
6. Ciccarello G, Mucciardi G, Morgia G, Spinelli F, Ascenti G, Macchione L, et al. A case of renal capsular liposarcoma with intracaval fat thrombus. Eur Urol. 2010; 57:350–353.
7. Dalal KM, Antonescu CR, Singer S. Diagnosis and management of lipomatous tumors. J Surg Oncol. 2008; 97:298–313.
8. Zuetenhorst JM, Taal BG. Metastatic carcinoid tumors: a clinical review. Oncologist. 2005; 10:123–131.
9. Osamura RY, Inomoto C, Kajiwara H, DeLellis RA. Neuroendocrine carcinomas of diverse sites. Pathol Case Rev. 2006; 11:282–291.
10. Romero FR, Rais-Bahrami S, Permpongkosol S, Fine SW, Kohanim S, Jarrett TW. Primary carcinoid tumors of the kidney. J Urol. 2006; 176(6 Pt 1):2359–2366.
11. Kawajiri H, Onoda N, Ohira M, Nakatani T, Wakasa K, Ishikawa T, et al. Carcinoid tumor of the kidney presenting as a large abdominal mass: report of a case. Surg Today. 2004; 34:86–89.
12. Kendal WS. The comparative survival of renal leiomyosarcoma. Can J Urol. 2007; 14:3435–3442.
13. Katabathina VS, Vikram R, Nagar AM, Tamboli P, Menias CO, Prasad SR. Mesenchymal neoplasms of the kidney in adults: imaging spectrum with radiologic-pathologic correlation. Radiographics. 2010; 30:1525–1540.
14. Grasso M, Blanco S, Fortuna F, Crippa S, Di Bella C. Spontaneous rupture of renal leiomyosarcoma in a 45-year-old woman. Arch Esp Urol. 2004; 57:870–872.
15. Ochiai K, Onitsuka H, Honda H, Kawamoto K, Uozumi J, Kumazawa J, et al. Leiomyosarcoma of the kidney: CT and MR appearance. J Comput Assist Tomogr. 1993; 17:656–658.
16. Lalwani N, Prasad SR, Vikram R, Katabathina V, Shanbhogue A, Restrepo C. Pediatric and adult primary sarcomas of the kidney: a cross-sectional imaging review. Acta Radiol. 2011; 52:448–457.
17. Zakhary MM, Elsayes KM, Platt JF, Francis IR. Magnetic resonance imaging features of renal synovial sarcoma: a case report. Cancer Imaging. 2008; 8:45–47.
18. Gong J, Kang W, Li S, Yang Z, Xu J. CT findings of synovial sarcomas of the kidney with pathological correlation. Clin Imaging. 2013; 37:1033–1036.
19. Murphey MD, Gibson MS, Jennings BT, Crespo-Rodríguez AM, Fanburg-Smith J, Gajewski DA. From the archives of the AFIP: imaging of synovial sarcoma with radiologic-pathologic correlation. Radiographics. 2006; 26:1543–1565.
20. Froehner M, Manseck A, Haase M, Hakenberg OW, Wirth MP. Locally recurrent malignant fibrous histiocytoma: a rare and aggressive genitourinary malignancy. Urol Int. 1999; 62:164–170.
21. Shirkhoda A, Lewis E. Renal sarcoma and sarcomatoid renal cell carcinoma: CT and angiographic features. Radiology. 1987; 162:353–357.
22. Eroğlu M, Bakirtaş H, Cimentepe E, Unsal A, Ataoğlu O, Balbay MD. Malignant fibrous histiocytoma arising from the renal capsule. Urol Int. 2005; 75:368–370.
23. Kearney MM, Soule EH, Ivins JC. Malignant fibrous histiocytoma: a retrospective study of 167 cases. Cancer. 1980; 45:167–178.
24. Kitajima K, Kaji Y, Morita M, Okuda Y, Sugimura K. Malignant fibrous histiocytoma arising from the renal capsule. Magn Reson Med Sci. 2003; 2:199–202.
25. Venter A, Roşca E, Muţiu G, Daina L, Pirte A. Difficulties of diagnosis in retroperitoneal tumors. Rom J Morphol Embryol. 2013; 54:451–456.
26. Sanyal R, Remer EM. Radiology of the retroperitoneum: case-based review. AJR Am J Roentgenol. 2009; 192:6 Suppl. S112–S117. Quiz S118-S121.
27. Chaudhari S, Hatwal D, Suri V. A rare case of primary fibrosarcoma of kidney. Iran J Kidney Dis. 2013; 7:67–69.
28. Funston MR, Levine E, Stables DP. Spontaneous renal hemorrhage. Urology. 1976; 8:610–617.
29. Raby WN, Kopplin P, Weitzman S. Metastatic osteosarcoma of the kidney presenting as renal hemorrhage. J Pediatr Hematol Oncol. 1996; 18:321–322.
30. Jeffree GM, Price CH, Sissons HA. The metastatic patterns of osteosarcoma. Br J Cancer. 1975; 32:87–107.
31. Caoili EM, Davenport MS. Role of percutaneous needle biopsy for renal masses. Semin Intervent Radiol. 2014; 31:20–26.
32. Lee SW, Lee MH, Yang HJ, Yang WJ, Kim DS, Lee NK, et al. Experience of ultrasonography-guided percutaneous core biopsy for renal masses. Korean J Urol. 2013; 54:660–665.
33. Volpe A, Mattar K, Finelli A, Kachura JR, Evans AJ, Geddie WR, et al. Contemporary results of percutaneous biopsy of 100 small renal masses: a single center experience. J Urol. 2008; 180:2333–2337.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download