INTRODUCTION
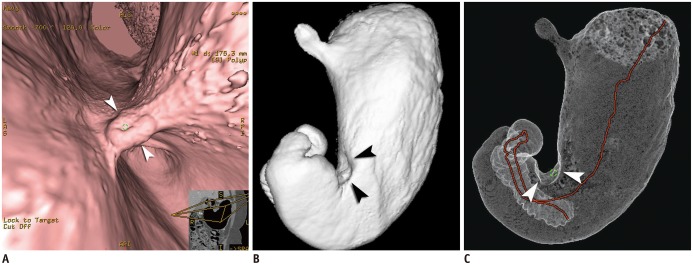 | Fig. 1Case of Borrmann type II advanced gastric cancer (AGC) visualized on various three-dimensional CT gastrography images.
Virtual endoscopy (VE) (A), shaded surface display (SSD) (B), and tissue transition projection (TTP) (C) images show Borrmann type II AGC (arrowheads) at gastric angle. As compared to SSD image, central ulceration of mass is depicted more clearly on VE image. Note that interactive two-dimensional image is concomitantly displayed in corner of VE image. Red line on TTP image indicates viewpoint path, along which center of VE image is located.
|
Technical Factors in MDCT Gastrography
CT Examination
Post-Processing
Evaluation Steps of MDCT Gastrographic Images
 | Fig. 2Various features of early gastric cancers (EGCs) on virtual endoscopy and conventional endoscopic images.
A. EGC (type IIb, superficial flat type) is seen with uneven base (arrows). B. EGC (type IIc, superficial depressed type) shows shallow depressed lesion with abnormal gastric fold convergence (arrows). C. EGC (type IIa, superficial elevated type) is demonstrated as bulbous enlargement (arrows). D. EGC (type I, protruded type) is seen as polypoid lesion (arrows).
|
Detection and Localization of Gastric Cancer
Detectability of Gastric Cancer Using MDCT Gastrography
Importance of Preoperative Localization of Gastric Cancer in Terms of Surgical Margin
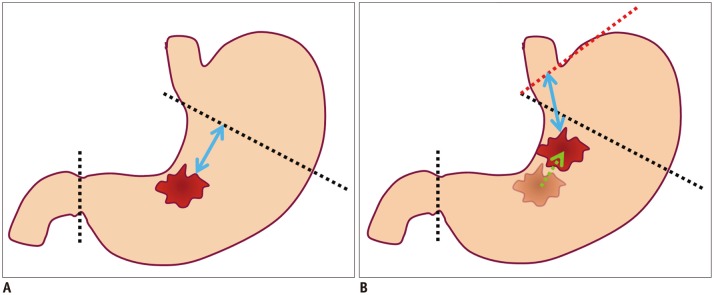 | Fig. 3Diagrams showing possibility of re-operation due to location difference for gastric cancer between preoperative conventional endoscopy and surgery.
A. Illustration shows location of gastric cancer at gastric angle, as is determined using preoperative conventional endoscopy. Black dotted lines indicate proximal and distal resection lines of planned laparoscopic subtotal gastrectomy with sufficient proximal resection margin (blue arrow). B. Illustration shows different location of gastric cancer between preoperative conventional endoscopy and surgery. If gastric cancer is located more proximally (green arrow) along lesser curvature at surgery than conventional endoscopy, planned proximal and distal resection lines (black dotted lines) would not secure sufficient proximal resected margin. Thus, in this case, additional total gastrectomy (red dotted line) may be required for sufficient proximal resected margin (blue arrow) after primary subtotal gastrectomy.
|
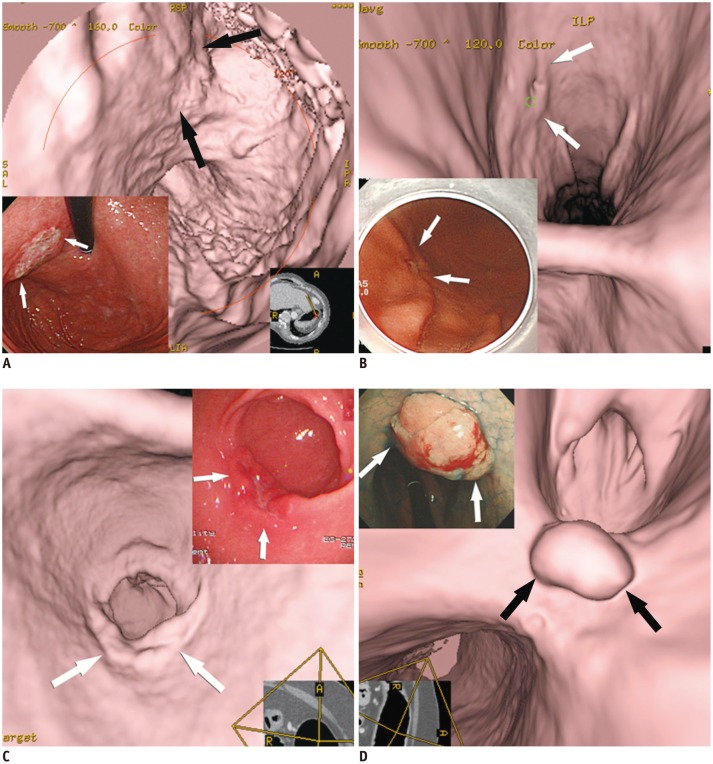
 | Fig. 4Case of early gastric cancer (EGC) (type IIa) in which re-operation was performed because location of EGC was incorrectly determined by conventional endoscopy, in contrast to CT gastrography.
A. EGC (arrows) was determined to be at lesser curvature of gastric mid-body using conventional endoscopy. Laparoscopic distal gastrectomy was planned based on conventional endoscopic findings. B, C. In contrast to conventional endoscopic findings, EGC (arrow) is depicted at upper body on both virtual endoscopy (B) and tissue transition projection (C) images. However, planned laparoscopic distal gastrectomy was finally performed according to location of EGC using conventional endoscopy. With surgical specimen, EGC was confirmed to be located at upper body. Re-operation (near-total gastrectomy) was subsequently performed due to insufficient proximal resected margin after initial laparoscopic distal gastrectomy.
|
T Staging of Gastric Cancer
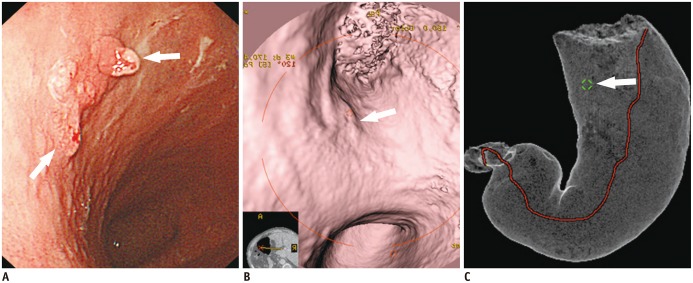
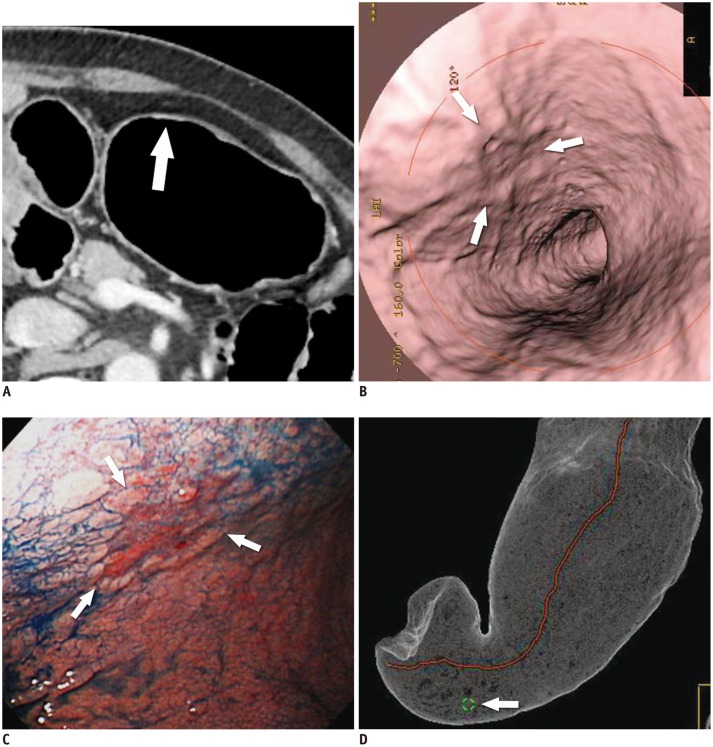 | Fig. 5T1a cancer (type IIc) in 62-year-old man that is not seen on two-dimensional CT image despite being detected on both virtual endoscopy (VE) and tissue transition projection (TTP) images.
A. Oblique, axial, contrast-enhanced CT image shows no discernible lesion at corresponding site (arrow) where early gastric cancer (EGC) is detected on three-dimensional images. B. VE image demonstrates shallow depressed lesion (arrows) with converging folds and uneven margins. C. Conventional endoscopic image shows malignant ulcer (arrows) with converging folds and uneven margin, which are similar morphologic features with B. D. TTP image depicts location of EGC (arrow) that is seen on VE (B).
|
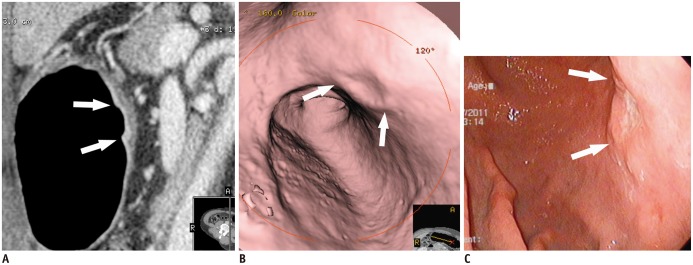 | Fig. 6T1b cancer (type IIa + IIc) in 62-year-old man, detected on both two-dimensional and three-dimensional (virtual endoscopy [VE]) images.
A. Sagittal contrast-enhanced CT image shows well-enhanced ulcerative lesion in thickened mucosal layer (arrows), which invades low-density-stripe layer to degree of less than 50% of thickness at lesser curvature of gastric lower body. B. VE image demonstrates shallow ulcerative lesion (arrows) with uneven margins. C. Conventional endoscopic image shows malignant ulcer (arrows) with uneven margins, similar to morphological features in B.
|
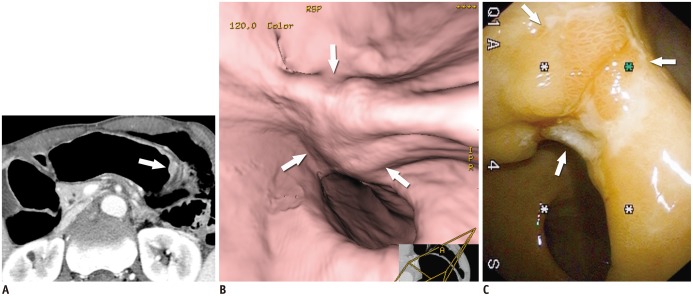 | Fig. 7T2 cancer in 69-year-old man.
A. Axial contrast-enhanced CT image shows well-enhancing mucosal thickening (arrow) and disruption of low-density-stripe layer (> 50% of thickness) at greater curvature of gastric body, without abutting outer, slightly higher-attenuating layer. This lesion was correctly classified as T2 cancer in preoperative imaging. B. Virtual endoscopy image shows ulceroinfiltrative mass (arrows). C. Conventional endoscopic image reveals poorly demarcated ulceroinfiltrative lesion (arrows), suggestive of advanced gastric cancer.
|
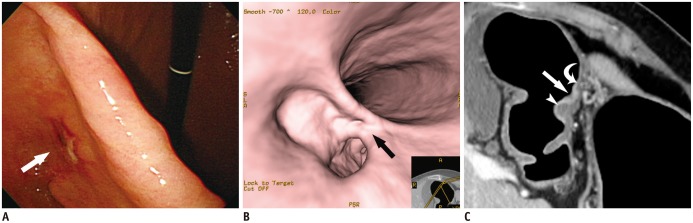 | Fig. 8T1b early gastric cancer (type IIc) in 74-year-old woman that was overestimated as T2 cancer at multidetector CT gastrography.
A, B. Conventional endoscopic (A) and virtual endoscopy (VE) (B) images clearly show focal ulcerative lesion (arrow). C. Oblique axial contrast-enhanced CT image shows focal ulcerative lesion with enhancing thickened mucosa (arrow) and uneven gastric layers in thickness. Because of relatively thin low-density-stripe layer (curved arrow) of proximal antrum, compared to distal antrum (arrowhead), disruption degree of low-density-stripe layer was estimated as greater than 50% of thickness. This lesion was judged preoperatively as T2 cancer. However, gastric cancer was confirmed pathologically as SM3 T1b cancer.
|
Limitations and Diagnostic Pitfalls of 3D MDCT Gastrography
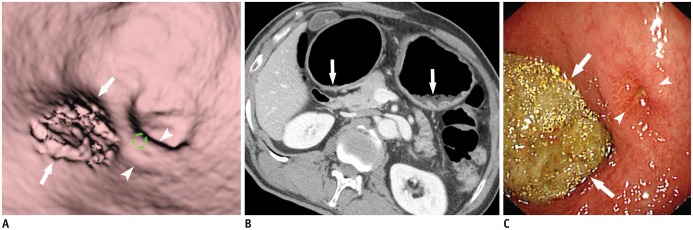 | Fig. 9Residual food mimicking true gastric lesion.
A. Residual food (arrows) in gastric antrum mimics focal mucosal lesion. Note tiny ulcer (arrowheads) in vicinity of residual food. B. Axial CT image demonstrates residual food (arrows) in gastric antrum and upper body. C. Conventional endoscopy reveals residual food (arrows) and tiny ulcer (arrowheads) in gastric antrum.
|




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download