Abstract
Objective
Although tuberculous lymphadenitis and Kikuchi disease are common causes of cervical lymphadenopathy in Asians and exhibit similar clinical manifestations, their treatment strategies are totally different. The purpose of this study was to identify ultrasonographic features that distinguish these two diseases.
Materials and Methods
This study was approved by the Institutional Review Board. The study included 77 patients with tuberculous lymphadenitis and 135 patients with Kikuchi disease. The sex and age distributions of the patients were analyzed. The size and shape of lymph nodes (LNs), presence of conglomeration, increased perinodal echogenicity, echogenic hilum, posterior neck involvement, internal calcification, patterns of internal necrosis, laterality of involved LNs, and hilar vascular patterns on ultrasonography were compared between the two groups. Multiple logistic regression analysis was conducted to identify independent findings to discriminate tuberculous lymphadenitis from Kikuchi disease. Finally, diagnostic accuracies were calculated using the independent findings.
Results
The presence of an echogenic hilum, internal calcification, patterns of internal necrosis, and LN hilar vascular structures on power Doppler ultrasonography were independent findings that discriminated tuberculous lymphadenitis from Kikuchi disease. The diagnostic accuracy of each of these four factors was 84.9% (181/212), 76.9% (163/212), 84% (178/212), and 89.2% (189/212), respectively. A combination of internal calcification and hilar vascular structures showed the best accuracy of 89.6% (190/212) (sensitivity, 86.7% [117/135]; specificity, 94.8% [73/77]) for diagnosing Kikuchi disease.
Tuberculous lymphadenitis and Kikuchi disease (histiocytic necrotizing lymphadenitis) are among the many causes of cervical lymphadenopathy, particularly in young Asian women. Even in Western countries, knowledge of tuberculosis is becoming more important because of the increasing number of patients taking immunosuppressants or who are infected with human immunodeficiency viruses. Young women who are dieting are particularly susceptible to tuberculous lymphadenitis. Several studies have reported that Kikuchi disease can affect anyone, regardless of age, sex, or ethnicity (12345). Tuberculous lymphadenitis and Kikuchi disease share certain clinical manifestations, such as cervical lymph node enlargement, low-grade fever, malaise, night sweats, and leucopenia (124567891011). They also share some pathological features, including necrotizing lymphadenitis (1213). However, the clinical course and treatment strategies for these two diseases are totally different. Tuberculous lymphadenitis requires long-term anti-tuberculosis treatment and may require surgical excision in severe cases, whereas Kikuchi disease is usually self-limiting and resolves spontaneously within 1-4 months (31415). Therefore, it is crucial to obtain a correct diagnosis early in the clinical course. Pathology can confirm the disease before treatment; however, the results of fine needle aspiration cytology may produce equivocal results due to the overlapping cytological features of the two diseases (1213). In addition, core needle biopsies (CNB) are occasionally very difficult to perform, particularly for small lymph nodes (< 1.5 cm) adjacent to vascular structures or when the physician is unfamiliar with the procedure. Therefore, ultrasonographic (US) evaluation of cervical lymphadenopathy, which is the primary noninvasive diagnostic modality, is important.
Although several reports have describing US findings of tuberculous lymphadenitis or Kikuchi disease, and one report compared computed tomography (CT) findings of tuberculous lymphadenitis and Kikuchi disease (16), no study has directly compared US findings of tuberculous lymphadenitis with those of Kikuchi disease in a large number of cases with respect to diagnostic accuracy. Therefore, the purpose of this study was to identify gray-scale and power Doppler US findings that differentiate these two diseases.
This retrospective study was approved by the Institutional Review Board, and informed consent was waived.
A total of 642 patients underwent US-guided CNB of neck lymph nodes at Korea University Guro Hospital from January 2010 to April 2014. Among them, 75 patients had reactive hyperplasia as a pathological result, 135 had Kikuchi disease, 77 had tuberculous lymphadenitis, 118 had metastatic lymphadenopathy, 74 had malignant lymphoma, and the remaining 163 patients had various other pathological findings.
A total of 212 patients with pathologically proven tuberculous lymphadenitis (n = 77) or Kikuchi disease (n = 135) were included in this study. Of these, 141 patients were female and 71 were male, and their mean age was 32.7 ± 16.6 years (range, 2-87 years).
All 212 patients underwent gray-scale US and a power Doppler study of the neck using high-resolution US units and high-frequency linear transducers (HDI5000 and iU22; Philips Healthcare, Bothell, WA, USA; LOGIQ9; GE Healthcare, Milwaukee, WI, USA). Two experienced head and neck radiologists performed US-guided CNB on the most representative node using a disposable 18-gauge gun biopsy needle (TSK Ace-cut; Create Medic, Yokohama, Japan or Angiotech; Medical Device Technologies, Inc., Gainesville, FL, USA). Power Doppler studies were performed with standardized power Doppler parameters set to high sensitivity and a low wall filter to allow detection of blood vessels with weaker blood flow.
Ultrasonographic findings of pathologically confirmed neck lymph nodes evaluated with gray-scale imaging included size (long and short diameters), shape (ratio of short diameter to long diameter), presence of conglomeration, increased perinodal echogenicity, echogenic hilum, posterior neck involvement (level V), internal calcification, patterns of internal necrosis, and laterality of involved lymph nodes (unilateral or bilateral). The hilar vascular patterns were assessed on power Doppler imaging. All images were analyzed by two head and neck radiologists using the consensus method.
The size of a scanned lymph node was measured at the longest axis (long diameter) and the greatest diameter among the lines perpendicular to the long diameter (short diameter) on the same scan. Lymph node shape was assessed by the ratio of short diameter to long diameter. Conglomeration was defined as clustering of at least three lymph nodes resembling a bunch of grapes. Increased perinodal echogenicity was defined as increased echogenicity surrounding a lymph node compared to adjacent perinodal fat tissues (Figs. 1, 2). Internal necrosis was defined as a marked hypoechoic portion in the lymph node without color flow on the Doppler study; if the necrotic component was less than approximately one-fourth of the lymph node, it was considered to be a partially necrotic lymph node; otherwise it was considered a gross necrotic lymph node (Fig. 3). The vascular patterns of lymph nodes were evaluated using power Doppler US. Hilar vascular patterns were divided into three groups according to the location of the hilar vascular structures: normal, in which hilar vascular structures were seen as central or branching radially from the hilum in both longitudinal and transverse planes; displaced, in which hilar vascular structures were seen in eccentric or asymmetric locations; and avascular, in which no hilar vascularity was seen on power Doppler US.
All statistical analyses were performed using MedCalc software for Windows (MedCalc software V, 12.6.1.0, Mariakerke, Belgium). A two-tailed p value ≤ 0.05 was considered significant for all statistical analyses. Patient age, lymph node size (long and short diameters), and lymph node shape (ratio of short diameter to long diameter) were compared between the two groups (tuberculous lymphadenitis and Kikuchi disease) using the unpaired Student's t test. Sex, presence of conglomeration, increased perinodal echogenicity, echogenic hilum, posterior neck involvement, patterns of internal necrosis, and laterality of involved lymph nodes (unilateral or bilateral) were compared between the two groups using the χ2 test. The presence of internal calcification was compared between the two groups using the Fisher's exact test.
We also conducted a multiple logistic regression analysis to determine which factors independently discriminated tuberculous lymphadenitis from Kikuchi disease among the factors with a p value < 0.1 in a univariate analysis. Among the factors, patterns of internal necrosis and vascular structure were dichotomously analyzed; presence or absence of necrosis, normal or abnormal vascular structure. All factors with a p value < 0.05 were considered independent factors to discriminate between the two diseases. We used the independent factors to calculate diagnostic accuracies, sensitivities, and specificities.
No significant difference was observed in the sex distribution between patients with tuberculous lymphadenitis (male/female = 26/51) and those with Kikuchi disease (male/female = 45/90; p = 0.931). However, a significant difference was detected in the age distribution between those with tuberculous lymphadenitis (mean age, 45.6 ± 15.2 years; range, 16-87 years) and those with Kikuchi disease (mean age, 25.3 ± 12.3 years; range, 2-66 years; p < 0.001).
The comparison of US findings between tuberculous lymphadenitis and Kikuchi disease is summarized in Table 1. In the size analysis, both the mean long and short diameters of lymph nodes were significantly greater in the tuberculous lymphadenitis group (2.3 ± 0.9 cm and 1.4 ± 0.6 cm, respectively) than those in the Kikuchi group (1.8 ± 0.6 cm and 0.9 ± 0.3 cm, respectively; all p < 0.001).
The mean ratio of the short diameter to long diameter in the tuberculous lymphadenitis group (0.6 ± 0.1) was significantly larger than that of the Kikuchi disease group (0.5 ± 0.1; p < 0.001). Thus, the lymph nodes in patients with tuberculous lymphadenitis showed a more rounded shape than those in patients with Kikuchi disease.
No significant differences were observed in the gray-scale US findings between the tuberculous lymphadenitis and Kikuchi disease groups in terms of presence of conglomeration, increased perinodal echogenicity (Figs. 1, 2), or laterality of involved lymph nodes (p = 0.062, 0.839, and 0.269, respectively).
However, a significantly smaller proportion of lymph nodes in the tuberculous lymphadenitis group had an echogenic hilum (24.7% [19/77] compared with 90.4% [122/135] in the Kikuchi disease group; p < 0.001) (Figs. 3, 4). The Kikuchi disease group showed a higher rate of posterior neck involvement (74.1% [100/135]) than that in the tuberculous lymphadenitis group (58.4% [45/77]; p = 0.028). Approximately 36.4% (28/77) of all lymph nodes in the tuberculous lymphadenitis group had internal calcification, whereas none of the lymph nodes in the Kikuchi disease group had internal calcification (p < 0.001) (Fig. 5). Furthermore, the internal necrosis patterns were significantly different between the two groups (p < 0.001) (Fig. 3). Fifty-five of 77 patients with tuberculous lymphadenitis (71.4%) had necrotic lymph nodes, and the majority of these (67.3% [37/55]) were gross necrotic nodes. In contrast, only 12 of 135 patients with Kikuchi disease (8.9%) had necrotic lymph nodes, and most of these (91.7% [11/12]) were partially necrotic.
Hilar vascular patterns on the power Doppler US were significantly different between the tuberculous lymphadenitis and the Kikuchi disease groups (p < 0.001) (Figs. 3, 4). The majority of the lymph nodes in the tuberculous lymphadenitis group had an avascular pattern (77.9% [60/77]), and 15.6% (12/77) of lymph nodes had a displaced hilar vascular pattern. Only 6.5% (5/77) of lymph nodes in the tuberculous lymphadenitis group showed a normal vascular pattern on power Doppler US. In contrast, the majority of lymph nodes in the Kikuchi disease group showed a normal hilar vascular pattern on power Doppler US (86.7% [117/135]), and a displaced hilar vascular pattern or avascular pattern was seen in only 8.9% (12/135) and 4.4% (6/135) of the lymph nodes, respectively.
A multiple logistic regression analysis including factors with a p value < 0.1 in the univariate analysis showed that patient age, presence of an echogenic hilum, internal calcification, pattern of internal necrosis, and hilar vascular structure were able to differentiate tuberculous lymphadenitis lymph nodes from Kikuchi lymph nodes. The size and shape of lymph nodes, presence of conglomeration, and posterior neck involvement were not independent factors. These results, with adjusted odds ratios and 95% confidence intervals are shown in Table 2.
The diagnostic accuracy values of each of the four independent factors (presence of echogenic hilum, internal calcification, pattern of internal necrosis, and hilar vascular structures) were 84.9% (181/212), 76.9% (163/212), 84% (178/212), and 89.2% (189/212), respectively. Sensitivities and specificities were 90.4% (122/135) and 75.3% (58/77) for the presence of an echogenic hilum, 100% (135/135) and 36.4% (28/77) for internal calcification, 91.1% (123/135) and 71.4% (55/77) for pattern of internal necrosis, and 86.8% (117/135) and 93.5% (72/77) for hilar vascular structure, respectively. The best diagnostic performance was achieved with a combination of the presence of internal calcification and the hilar vascular structure pattern, which showed accuracy of 89.6% (190/212), sensitivity of 86.7% (117/135), and specificity of 94.8% (73/77) for diagnosing Kikuchi disease.
Our results show that the presence of an echogenic hilum, internal calcification, pattern of internal necrosis, and hilar vascular structure on power Doppler US independently distinguished tuberculous lymphadenitis from Kikuchi disease. The diagnostic accuracy of each of these four factors was approximately 80%, and the best diagnostic performance of 89.6% was achieved with the combination of internal calcification and hilar vascular structure pattern.
Sex and many other US findings, such as long and short diameters, lymph node shape, presence of posterior neck involvement, conglomeration, and laterality of the involved lymph nodes were not significant in the multiple logistic regression analysis. However, long and short diameters and lymph node shape were significantly different (p < 0.001) in the univariate analysis. Although these were not independent discriminators, those findings could be useful for distinguishing between the two diseases.
An echogenic hilum was observed in 90% of patients with Kikuchi disease compared with only approximately 25% of cases of tuberculous lymphadenitis. These values were slightly higher than those observed for Kikuchi disease in several other studies, which reported rates of 30-87% (171819), and lower than those reported previously for tuberculous lymphadenitis (35-70%) (122021).
Posterior neck involvement is common in patients with Kikuchi disease (22). However, a one study showed that posterior neck involvement is more frequently observed in patients with tuberculous lymphadenitis than those with Kikuchi disease (16). In our study, posterior neck involvement was present in 74% of patients with Kikuchi disease and 58% of patients with tuberculous lymphadenitis and was not an independent factor in multiple logistic regression analysis.
Approximately 36.4% of the tuberculous lymphadenitis cases had internal calcification, whereas no calcified lymph nodes were detected in the Kikuchi disease group. Previous studies also reported no internal calcification in patients with Kikuchi disease (1619), whereas the reported frequency of internal calcification in patients with tuberculous lymphadenitis is 24-84% (12161823). As strong echogenicity includes not only fibrocalcification, but also hyalinosis and artifacts resulting from caseous necrosis in tuberculous lymphadenitis, US findings usually show a higher rate of calcification than CT findings (1223). Indeed, we found that some lymph nodes with calcification on US showed no calcification on CT scans.
The patterns of internal necrosis were significantly different between the two groups. Approximately 71% of cases of tuberculous lymphadenitis had necrosis, the majority of which were gross necrotic nodes, whereas fewer than 10% of lymph nodes in patients with Kikuchi disease had a necrotic portion, and most of these had partial necrosis. These findings were largely consistent with those of previous studies (11619). However, studies that used CT showed a higher rate of necrosis than studies using US findings, probably because thick necrotic debris would be seen on US as a heterogeneous echoic lesion rather than a marked hypoechoic portion.
Lastly, hilar vascular patterns on power Doppler US were significantly different between the tuberculous lymphadenitis and Kikuchi disease groups. The majority of lymph nodes in the tuberculous lymphadenitis group (78%) had an avascular or displaced pattern (16%), whereas the majority of lymph nodes in the Kikuchi disease group (87%) had a normal hilar vascular pattern. These results are concordant with previous studies of tuberculous lymphadenitis or Kikuchi disease (192024). These displaced or absent vascular patterns are thought to be related to gross necrosis of lymph nodes in patients with tuberculous lymphadenitis.
Conglomeration is a typical finding in cases of tuberculous lymphadenitis as a result of periadenitis and perinodal soft tissue edema (121723). In the present study, tuberculous lymphadenitis tended to have a higher rate of conglomeration (30%) than that of Kikuchi disease (18%), but the difference was not significant (p = 0.062). This result could be related to lymph node conglomeration caused by the perinodal inflammatory reaction that can occur in patients with Kikuchi disease (72526). The lower rates of conglomeration in our two groups compared with those in previous studies may be related to the definition of conglomeration, which we defined as at least three lymph nodes agglomerated, like a bunch of grapes, whereas other studies usually defined conglomeration as a fusion of at least two lymph nodes (121723).
The structures surrounding the lymph nodes in patients with Kikuchi disease are infiltrated by attenuating perivascular and interstitial inflammatory cells, including a mixture of lymphoid cells, histiocytes, and characteristic karyorrhectic debris (31719). Increased perinodal echogenicity or attenuation is a characteristic finding of Kikuchi disease (1216172728). In our study, > 76% of lymph nodes in patients with Kikuchi disease showed increased perinodal echogenicity. However, patients with tuberculous lymphadenitis also showed increased perinodal echogenicity (74%). Such findings have been reported by previous studies on tuberculous lymphadenitis and were attributed to periadenitis caused by destruction of the lymph node capsule in active-stage tuberculosis (12293031).
Some limitations of our study should be discussed. First, it was a retrospective study. Second, there may have been selection bias, as only patients who underwent US and US-guided CNB were included. Third, the lymph nodes evaluated for posterior neck involvement and laterality of involved lymph nodes were not pathologically confirmed. We assumed that lymph nodes with gray-scale US findings that were similar to those of biopsied nodes would have the same pathology and evaluated these. However, our results show that laterality and presence of posterior neck involvement were not significant factors in the multiple logistic regression analysis. Fourth, three histological types (proliferative, necrotizing, and xanthomatous) are recognized in Kikuchi disease (61932), and US findings may differ according to histologic type. We did not evaluate US findings according to the Kikuchi disease subtype; however, these pathological types are considered to be serial evolving phases rather than independent pathological subtypes and are not thought to affect the prognosis or treatment decision (3233). Last, this study has the fundamental limitation of US evaluation caused by subjectivity or inter-rater variability.
In conclusion, our results suggest that the US findings of presence of an echogenic hilum, internal calcification, pattern of internal necrosis, and lymph node hilar vascular structures can help differentiate tuberculous lymphadenitis from Kikuchi disease.
Figures and Tables
 | Fig. 154-year-old woman with tuberculous lymphadenitis.
A, B. Conglomerated cervical lymphadenopathies show increased perinodal echogenicity (arrows) on ultrasonography (A) and increased perinodal attenuation (white circle) on computed tomography scan (B).
|
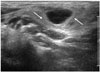 | Fig. 226-year-old woman with Kikuchi diseaseCervical lymph node shows increased perinodal echogenicity (arrows).
|
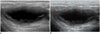 | Fig. 340-year-old woman diagnosed with tuberculous lymphadenitis.
A, B. Enlarged cervical lymph node shows gross internal necrosis and no definite echogenic hilum on gray-scale ultrasonography (A) and avascular pattern on power Doppler ultrasonography (B).
|
 | Fig. 437-year-old man diagnosed with Kikuchi disease.
A, B. Elongated cervical lymph node shows internal echogenic hilum (asterisk) and no definite necrotic component on gray-scale ultrasonography (A) and normal hilar vascular pattern on power Doppler ultrasonography (B). C. Axial computed tomography scan shows definite necrosis at same lymph node (arrow).
|
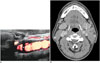 | Fig. 559-year-old man with tuberculous lymphadenitis.
A. Small cervical lymph node shows internal hyperechoic lesions (arrows) with some posterior acoustic shadowing (asterisk) interpreted as calcification. B. No calcification was detected on computed tomography scan of same lymph nodes (arrow).
|
Table 1
Comparison of Ultrasonographic Findings between TL and KD
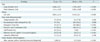
Table 2
Multiple Logistic Regression Analysis to Determine Independent Ultrasonographic Findings to Discriminate Tuberculous Lymphadenitis from Kikuchi Disease

References
1. Kwon SY, Kim TK, Kim YS, Lee KY, Lee NJ, Seol HY. CT findings in Kikuchi disease: analysis of 96 cases. AJNR Am J Neuroradiol. 2004; 25:1099–1102.
2. Na DG, Chung TS, Byun HS, Kim HD, Ko YH, Yoon JH. Kikuchi disease: CT and MR findings. AJNR Am J Neuroradiol. 1997; 18:1729–1732.
3. Onciu M, Medeiros LJ. Kikuchi-Fujimoto lymphadenitis. Adv Anat Pathol. 2003; 10:204–211.
4. Song JY, Lee J, Park DW, Sohn JW, Suh SI, Kim IS, et al. Clinical outcome and predictive factors of recurrence among patients with Kikuchi's disease. Int J Infect Dis. 2009; 13:322–326.
5. Youk JH, Kim EK, Ko KH, Kim MJ. Sonographic features of axillary lymphadenopathy caused by Kikuchi disease. J Ultrasound Med. 2008; 27:847–853.
6. Bosch X, Guilabert A, Miquel R, Campo E. Enigmatic Kikuchi-Fujimoto disease: a comprehensive review. Am J Clin Pathol. 2004; 122:141–152.
7. Han HJ, Lim GY, Yeo DM, Chung NG. Kikuchi's disease in children: clinical manifestations and imaging features. J Korean Med Sci. 2009; 24:1105–1109.
8. Fontanilla JM, Barnes A, von Reyn CF. Current diagnosis and management of peripheral tuberculous lymphadenitis. Clin Infect Dis. 2011; 53:555–562.
9. Artenstein AW, Kim JH, Williams WJ, Chung RC. Isolated peripheral tuberculous lymphadenitis in adults: current clinical and diagnostic issues. Clin Infect Dis. 1995; 20:876–882.
10. Khan FY. Clinical pattern of tuberculous adenitis in Qatar: experience with 35 patients. Scand J Infect Dis. 2009; 41:128–134.
11. Wei YF, Liaw YS, Ku SC, Chang YL, Yang PC. Clinical features and predictors of a complicated treatment course in peripheral tuberculous lymphadenitis. J Formos Med Assoc. 2008; 107:225–231.
12. Khanna R, Sharma AD, Khanna S, Kumar M, Shukla RC. Usefulness of ultrasonography for the evaluation of cervical lymphadenopathy. World J Surg Oncol. 2011; 9:29.
13. Tong TR, Chan OW, Lee KC. Diagnosing Kikuchi disease on fine needle aspiration biopsy: a retrospective study of 44 cases diagnosed by cytology and 8 by histopathology. Acta Cytol. 2001; 45:953–957.
14. Lin HC, Su CY, Huang CC, Hwang CF, Chien CY. Kikuchi's disease: a review and analysis of 61 cases. Otolaryngol Head Neck Surg. 2003; 128:650–653.
15. Poulose V, Chiam P, Poh WT. Kikuchi's disease: a Singapore case series. Singapore Med J. 2005; 46:229–232.
16. Lee S, Yoo JH, Lee SW. Kikuchi disease: differentiation from tuberculous lymphadenitis based on patterns of nodal necrosis on CT. AJNR Am J Neuroradiol. 2012; 33:135–140.
17. Lo WC, Chang WC, Lin YC, Hsu YP, Liao LJ. Ultrasonographic differentiation between Kikuchi's disease and lymphoma in patients with cervical lymphadenopathy. Eur J Radiol. 2012; 81:1817–1820.
18. Ying M, Ahuja AT, Yuen HY. Grey-scale and power Doppler sonography of unusual cervical lymphadenopathy. Ultrasound Med Biol. 2004; 30:449–454.
19. Yoo JL, Suh SI, Lee YH, Seo HS, Kim KM, Shin BK, et al. Gray scale and power Doppler study of biopsy-proven Kikuchi disease. J Ultrasound Med. 2011; 30:957–963.
20. Ahuja A, Ying M, King A, Yuen HY. Lymph node hilus: gray scale and power Doppler sonography of cervical nodes. J Ultrasound Med. 2001; 20:987–992. quiz 994
21. Ahuja AT, Ying M. Sonographic evaluation of cervical lymph nodes. AJR Am J Roentgenol. 2005; 184:1691–1699.
22. Harnsberger HR, Glastonbury CM, Michel MA, Koch BL, Branstetter BF, Davidson HC, et al. Diagnostic Imaging: Head and Neck. 2nd ed. Frisens, Altona, Manitoba, Canada: Amirsys;2011.
23. Asai S, Miyachi H, Suzuki K, Shimamura K, Ando Y. Ultrasonographic differentiation between tuberculous lymphadenitis and malignant lymph nodes. J Ultrasound Med. 2001; 20:533–538.
24. Ying M, Ahuja A, Brook F. Accuracy of sonographic vascular features in differentiating different causes of cervical lymphadenopathy. Ultrasound Med Biol. 2004; 30:441–447.
25. Miller WT Jr, Perez-Jaffe LA. Cross-sectional imaging of Kikuchi disease. J Comput Assist Tomogr. 1999; 23:548–551.
26. Pombo F, Rodríguez E, Mato J, Pérez-Fontán J, Rivera E, Valvuena L. Patterns of contrast enhancement of tuberculous lymph nodes demonstrated by computed tomography. Clin Radiol. 1992; 46:13–17.
27. Fulcher AS. Cervical lymphadenopathy due to Kikuchi disease: US and CT appearance. J Comput Assist Tomogr. 1993; 17:131–133.
28. Ogawa M, Ueda S, Ohto M, Fujita M, Kubosawa T. Ultrasonography of cervical lymphadenopathy in a patient with Kikuchi's disease. Acta Radiol. 1991; 32:260–261.
29. De Backer AI, Mortelé KJ, Van Den Heuvel E, Vanschoubroeck IJ, Kockx MM, Van de Vyvere M. Tuberculous adenitis: comparison of CT and MRI findings with histopathological features. Eur Radiol. 2007; 17:1111–1117.
30. Lee Y, Park KS, Chung SY. Cervical tuberculous lymphadenitis: CT findings. J Comput Assist Tomogr. 1994; 18:370–375.
31. Moon WK, Han MH, Chang KH, Im JG, Kim HJ, Sung KJ, et al. CT and MR imaging of head and neck tuberculosis. Radiographics. 1997; 17:391–402.
32. Kuo TT, Lo SK. Significance of histological subtypes of Kikuchi's disease: comparative immunohistochemical and apoptotic studies. Pathol Int. 2004; 54:237–240.
33. Hutchinson CB, Wang E. Kikuchi-Fujimoto disease. Arch Pathol Lab Med. 2010; 134:289–293.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download