Abstract
Objective
This study was conducted to evaluate stent compression in iliac vein compression syndrome (IVCS) and to identify its association with stent patency.
Materials and Methods
Between May 2005 and June 2014, after stent placement for the treatment of IVCS with acute ilio-femoral deep vein thrombosis, follow-up CT venography was performed in 48 patients (35 women, 13 men; age range 23-87 years; median age 56 years). Using follow-up CT venography, the degree of the stent compression was calculated and used to divide patients into two groups. Possible factors associated with stent compression and patency were evaluated. The cumulative degree of stent compression and patency rate were analyzed.
Results
All of the stents used were laser-cut nitinol stents. The proportion of limbs showing significant stent compression was 33%. Fifty-six percent of limbs in the significant stent compression group developed stent occlusion. On the other hand, only 9% of limbs in the insignificant stent compression group developed stent occlusion. Significant stent compression was inversely correlated with stent patency (p < 0.001). The median patency period evaluated with Kaplan-Meier analysis was 20.0 months for patients with significant stent compression. Other factors including gender, age, and type of stent were not correlated with stent patency. Significant stent compression occurred most frequently (87.5%) at the upper end of the stent (ilio-caval junction).
Iliac vein compression syndrome (IVCS), also known as May-Thurner syndrome, is a syndrome resulting from compression of the left iliac vein by the overlying iliac artery (1). In recent decades, iliac vein stenting has demonstrated excellent long-term patency and clinical outcome (234).
Self-expanding stents have been widely used for the treatment of obstructive iliac venous disease. Self-expanding nitinol stents have advantages such as minimal foreshortening, better flexibility, and higher resistive force in comparison with Wallstents (5678). Various factors may influence stent patency in the venous system such as obstruction, location of the stent, amount of venous inflow, and presence of concomitant hypercoagulable disorder. However, the cause of stent occlusion is not always identified. In addition, long-term changes of stents in the venous system are unknown (9).
Occasionally, stent compression is observed on follow-up CT venography and, for this reason, some authors have suggested that laser-cut nitinol stents may not be suitable for treating IVCS with severe extrinsic compression (10). Therefore, the objectives of this study were to assess the characteristics of stent compression and its effect on stent patency by retrospectively analyzing CT venography after stent placement for IVCS.
This is a retrospective study conducted at a single tertiary-care academic center with internal review board approval. Between May 2005 and June 2014, ninety-eight limbs were treated for acute ilio-femoral deep vein thrombosis within 2 weeks of symptom onset due to compression of the left common iliac vein. All patients underwent aspiration thrombectomy, and balloon angioplasty followed by stent placement in the left common iliac vein. Catheter-directed thrombolysis was performed in seven patients who had residual thrombotic material causing limitation of blood flow after thrombectomy. A total of 98 baseline CT venographic examinations were performed on an multidetector CT scanner (Brilliance 64, Philips Healthcare, Best, The Netherlands). The parameters for CT venography were beam collimation, 64 × 0.625 mm; pitch 1:0.891; slice thickness 2 mm; and reconstruction interval, 1.25 mm. Images were obtained with the patient in the supine position.
Iliac vein compression syndrome was defined as the presence of extrinsic compression in the left common iliac vein by the overlying right common iliac artery on CT venography combined with extensive deep vein thrombosis of the left lower extremity. Acute deep vein thrombosis was identified based on the following findings on CT venography: presence of filling defect in an opacified vein, lack of visualization of a venous segment between opacified proximal and distal veins, intraluminal high attenuation on non-enhanced CT images, venous dilatation of vein, and diffuse soft-tissue infiltration in the lower extremity suggestive of edema (1112).
According to the inclusion criteria, all patients with deep vein thrombosis of the right lower extremity were excluded. Among these 98 limbs, fifty patients who did not undergo follow-up CT were excluded. As a result, a total 48 limbs among 48 patients (female, 73%; male, 27%) were included in this study. The median age was 56 years (range, 23-87 years).
The procedures were performed by two interventional radiologists with more than ten years of experience in endovascular therapy. All procedures were performed with the patient in the prone position. Under ultrasonographic guidance, the ipsilateral popliteal vein was accessed and a 12-Fr sheath was placed. After assessing the extent of venous thrombosis on ascending venography, the thrombus was manually aspirated through a 9-Fr introducer sheath (Super Arrow-Flex; Arrow international, Reading, PA, USA). For those who underwent catheter-directed thrombolysis, overnight infusion of urokinase (Urokinase-GCC, Green Cross, Yongin, Korea) was performed through a multiple side-hole infusion catheter (Multisideport Catheter, Cook, Bloomington, IN, USA). Thereafter, balloon angioplasty was performed in the stenotic iliac vein followed by stent placement. Venous stenting was performed regardless of the degree of residual stenosis after balloon angioplasty. After stenting, post-dilation was performed if there was evidence of waisting in the stent. The patency of the stents was confirmed by final venography after the procedure.
Self-expanding laser-cut nitinol stents were used in all cases. Smart (Cordis, Bridgewater, NJ, USA) stents were deployed in 25 patients, Zilver (Cook, Bloomington, IN, USA) stents in 7 patients, and Luminexx (Bard, Murray Hill, NJ, USA) stents in 16 patients. The mean diameter and length of the stents were 13.4 mm (range, 12-14 mm) and 8 cm (range, 4-14 cm), respectively. In most of the limbs (90%), a single stent was placed. Two stents were placed in five limbs due to insufficient stent length (Table 1). The stents were positioned so that the upper end of the stent protruded approximately 1.0 cm into the inferior vena cava (IVC).
All patients were administered with low-molecular-weight heparin until discharge and then converted to oral warfarin for a minimum of 6 months. The target therapeutic range for the international normalized ratio was 2-3.
On follow-up CT venography, the degree of stent compression was assessed by calculating the percentage of diameter reduction of the stent lumen (diameter of the stent at the narrowest segment/diameter of the stent at the widest segment × 100%). According to the degree of stent compression, the limbs were categorized into either "significant" (luminal collapse at 50% or more) or "insignificant" (luminal collapse under 50%) stent compression groups. Thirty-nine of 45 patients underwent follow-up CT venography, while the remaning six patients had CT scans (which included venous phase of contrast enhancement) that had been performed for other reasons such as abdominal or pelvic pain. Two interventional radiologists reviewed the CT venography images including multi-planar or curved planar reformations which had been acquired using software for three-dimensional reconstruction (Rapidia, Infinitt, Seoul, Korea). The location of stent compression was documented (Fig. 1).
The interval of follow-up CT varied among patients according to the discretion of the referring physician. The median time interval between the procedure and the first follow-up by CT venography was 17 months (range, 1-72 months). Twelve out of the 48 patients underwent further sequential follow-up by CT venography. The median time interval until the final follow-up by CT venography was 23 months (range, 1-84 months).
Categorical variables associated with stent compression or occlusion were compared using a univariate logistic regression model (χ2 test). Rates for primary patency and secondary patency were defined by the reporting standards of the Society for Vascular Surgery/International Society for Cardiovascular Surgery (13). A cumulative patency curve obtained using the Kaplan-Meier method was used for the analysis.
On follow-up CT, 16 of 48 cases (33%) demonstrated significant stent compression. The most common location of stent compression in this group was the upper end of the stent (87.5%) caused by the right common iliac artery. Less common locations included the lower end (6.25%) where the left internal iliac artery caused the compression. In others, the entire length of the stent (6.25%) was under-expanded due to diffuse stenosis of the left iliac vein. There was no evidence of stent fracture or migration. All stent compression was detected on the initial follow-up CT and the median time interval between the procedure and the first follow-up by CT venography was 17 months (range, 1-72 months), and the median time to discovery of stent compression was 21 months (range, 1-84 months). No other factors were associated with the degree of stent compression.
The primary and secondary patency rates were 72% and 85.7% at 24 months. During follow-up, 12 out of 48 stents (25%) became occluded after a median time period of 21 months after the procedure. Gender, age, presence of IVC thrombus, type of stent, length and diameter of the stents, and the number of stents did not affect stent patency.
However, stent occlusion developed more frequently in the significant stent compression group than in the insignificant stent compression group (56%, 9/16 vs. 9%, 3/32, p < 0.001) (Table 1). Based on the cumulative patency rate in limbs with significant stent collapse, the median time to occlusion was 20 months (Fig. 2).
Re-interventions were performed for 5 of 12 occluded limbs (41.6%). Thrombectomy and balloon angioplasty were performed in all cases. Additional stent placement was required in three cases and all additional stents were nitinol stents.
Laser-cut nitinol and stainless steel stents (Wallstent, Boston Scientific, Natick, MA, USA) are commonly used for treating iliac vein obstruction. Both stents have excellent long-term patency with good clinical outcomes (51415). However, there is further potential for improvement with currently available stents. In a previous report, Wallstent, a widely used stainless steel stent, demonstrated a tendency to migrate or to be compressed at the upper end, sometimes requiring re-intervention (16171819). In our study, we sought to assess luminal compression in nitinol stents used to treat IVCS which, to the best of our knowledge, has never been addressed.
In this study, significant compression of nitinol stents was demonstrated in about a third of the patients. More than half of the stents with significant compression eventually developed stent occlusion, whereas less than a tenth of stents with insignificant compression became occluded. Stent compression exceeding 50% of the luminal diameter was the only factor that was associated with the development of occlusion, demonstrating a strong impact on stent patency (p < 0.001). This finding indicates the need for stents with improved radial resistive force. Stent fracture was not observed in any of our cases indicating that nitinol stents possess sufficient fatigue resistance. Furthermore, no cases of stent migration were found.
Stent compression occurred most frequently (87.5%) at the upper end of the stent or near the ilio-caval junction. When compressed near the ilio-caval junction, the cylindrical form transformed into a cone figure at the narrowed ostia. Raju et al. (16) said that this is local migration and the lesion compresses the stent subsequently or during post-dilatation with recurred stenosis. Negus et al. (17) and Raju and Neglén (20) demonstrated that end-effects of stents were common when treating a lesion in this region, especially when an attempt was made to place the stent precisely at this point to avoid encroachment of the stent into the vena cava proper. Negus et al. also stated that constriction of the stent diameter by as much as 20-30% could occur due to recoil in tight lesions, resulting in unpredictable stent length after deployment. The other common locations were in the lower end (6.3%) or along the entire length of the stent (6.3%). In a case of compression in the lower end, stent compression resulted from crossing of the left internal iliac artery. These results suggest that compression by the left internal iliac artery can be an uncommon cause of significant stent compression, and can consequently affect stent patency. Precise stent deployment in such circumstances is not easy owing to the variability in the anatomy of the venous bifurcations, the location of the overlying iliac artery, and length of the stenotic lesion (1721). Venography has been demonstrated to be a poor tool for assessing such anatomy (22). To solve this problem, there are recommendations in the literature to place the stent so that the upper end is extended into the IVC (23). However, such recommendations have not been widely adopted because of concerns related to jailing of in-flow from the contra-lateral iliac vein (19). Neglén and Raju (22) demonstrated that intravascular ultrasound scan could be an invaluable aid in the accurate placement of stents in occluded veins.
In our study, there were twelve cases of stent occlusion, five of which underwent re-intervention. Additional stent placement was needed in three cases of stent occlusion associated with significant stent compression. Raju et al. (16) reported that about 20% of Wallstents that had been deployed in the iliac vein for IVCS eventually required re-intervention to address problems at the upper end of the stent or within the stent stack itself. In-stent restenosis or thrombus often occurs because of in-flow or out-flow problems at either end of the stent. According to the literature problems including stent compression at the upper end alone, or in combination with other malfunctions, occupies half or more of re-interventions.
Jeon et al. (10) reported that in-stent thrombosis occurred usually within 3 months after stent placement and they emphasized the importance of short-term follow-up. In line with their report, our study also revealed that all cases of stent occlusion associated with significant stent compression were detected on the initial follow-up CT performed after a medial time interval of 17 months after intervention. The findings suggest that severe extrinsic compression exceeding the radial resistive force of the stent at the ilio-caval junction may strongly affect the early outcome.
There are some limitations to this study. First, half of the patients that underwent stent placement were excluded from this study due to loss of follow-up. Second, because this study was retrospective in nature, there was a lack of uniformity in the interval until follow-up by CT and the number of times CT was performed.
In conclusion, our results suggest that significant compression of nitinol stents placed in IVCS is highly associated with the development of stent occlusion and usually demonstrated on CT performed during the early period of follow-up. Therefore, in order to prevent stent compression in IVCS, higher radial resistive force of nitinol stents may be needed. If significant stent compression occurs during follow-up, early re-intervention may help to achieve better outcomes with regards to stent patency.
Figures and Tables
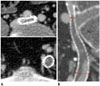 | Fig. 1Follow-up CT image shows significant stent compression and occlusion.On axial images, stent was compressed by crossing iliac artery at ilio-caval junction (A). On curved planar images, degree of stent compression was measured with caliper and calculated as diameter of stent at narrowest/widest point (bidirectional arrows) of stent × 100%. In this patient, stent was compressed more than 50% by crossing iliac artery at ilio-caval junction (B).
|
 | Fig. 2Kaplan-Meier curves show patency rates according to stent compression rate.Median patency period was 20.0 months for patients with significant stent compression (p = 0.001).
|
Table 1
Comparison between Significant Stent Compression and Insignificant Stent Compression According to Stent Occlusion

References
1. Mickley V, Schwagierek R, Rilinger N, Görich J, Sunder-Plassmann L. Left iliac venous thrombosis caused by venous spur: treatment with thrombectomy and stent implantation. J Vasc Surg. 1998; 28:492–497.
2. O'Sullivan GJ, Semba CP, Bittner CA, Kee ST, Razavi MK, Sze DY, et al. Endovascular management of iliac vein compression (May-Thurner) syndrome. J Vasc Interv Radiol. 2000; 11:823–836.
3. Mousa AY, AbuRahma AF. May-Thurner syndrome: update and review. Ann Vasc Surg. 2013; 27:984–995.
4. Kim JY, Choi D, Guk Ko Y, Park S, Jang Y, Lee do Y. Percutaneous treatment of deep vein thrombosis in May-Thurner syndrome. Cardiovasc Intervent Radiol. 2006; 29:571–575.
5. Stoeckel D, Pelton A, Duerig T. Self-expanding nitinol stents: material and design considerations. Eur Radiol. 2004; 14:292–301.
6. Duda SH, Wiskirchen J, Tepe G, Bitzer M, Kaulich TW, Stoeckel D, et al. Physical properties of endovascular stents: an experimental comparison. J Vasc Interv Radiol. 2000; 11:645–654.
7. Neglén P, Tackett TP Jr, Raju S. Venous stenting across the inguinal ligament. J Vasc Surg. 2008; 48:1255–1261.
8. Kurklinsky AK, Bjarnason H, Friese JL, Wysokinski WE, McBane RD, Misselt A, et al. Outcomes of venoplasty with stent placement for chronic thrombosis of the iliac and femoral veins: single-center experience. J Vasc Interv Radiol. 2012; 23:1009–1015.
9. Knipp BS, Ferguson E, Williams DM, Dasika NJ, Cwikiel W, Henke PK, et al. Factors associated with outcome after interventional treatment of symptomatic iliac vein compression syndrome. J Vasc Surg. 2007; 46:743–749.
10. Jeon UB, Chung JW, Jae HJ, Kim HC, Kim SJ, Ha J, et al. May-Thurner syndrome complicated by acute iliofemoral vein thrombosis: helical CT venography for evaluation of long-term stent patency and changes in the iliac vein. AJR Am J Roentgenol. 2010; 195:751–757.
11. Baldt MM, Zontsich T, Stümpflen A, Fleischmann D, Schneider B, Minar E, et al. Deep venous thrombosis of the lower extremity: efficacy of spiral CT venography compared with conventional venography in diagnosis. Radiology. 1996; 200:423–428.
12. Chung JW, Yoon CJ, Jung SI, Kim HC, Lee W, Kim YI, et al. Acute iliofemoral deep vein thrombosis: evaluation of underlying anatomic abnormalities by spiral CT venography. J Vasc Interv Radiol. 2004; 15:249–256.
13. Porter JM, Moneta GL. Reporting standards in venous disease: an update. International Consensus Committee on Chronic Venous Disease. J Vasc Surg. 1995; 21:635–645.
14. DeRubertis BG, Alktaifi A, Jimenez JC, Rigberg D, Gelabert H, Lawrence PF. Endovascular management of nonmalignant iliocaval venous lesions. Ann Vasc Surg. 2013; 27:577–586.
15. Neglén P. Stenting is the "Method-of-Choice" to treat iliofemoral venous outflow obstruction. J Endovasc Ther. 2009; 16:492–493.
16. Raju S, Tackett P Jr, Neglen P. Reinterventions for nonocclusive iliofemoral venous stent malfunctions. J Vasc Surg. 2009; 49:511–518.
17. Negus D, Fletcher EW, Cockett FB, Thomas ML. Compression and band formation at the mouth of the left common iliac vein. Br J Surg. 1968; 55:369–374.
18. Lugli M, Maleti O. Preliminary report on a new concept stent prototype designed for venous implant. Phlebology. 2014; 06. 11. pii: 0268355514539680. [Epub].
19. Raju S, Ward M Jr, Kirk O. A modification of iliac vein stent technique. Ann Vasc Surg. 2014; 28:1485–1492.
20. Raju S, Neglén P. Percutaneous recanalization of total occlusions of the iliac vein. J Vasc Surg. 2009; 50:360–368.
21. Raju S, Neglen P. High prevalence of nonthrombotic iliac vein lesions in chronic venous disease: a permissive role in pathogenicity. J Vasc Surg. 2006; 44:136–143. discussion 144
22. Neglén P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. J Vasc Surg. 2002; 35:694–700.
23. Raju S, McAllister S, Neglen P. Recanalization of totally occluded iliac and adjacent venous segments. J Vasc Surg. 2002; 36:903–911.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download