Abstract
Adenosine is a short-acting coronary vasodilator, and it is widely used during pharmacological stress myocardial perfusion imaging. It has a well-established safety profile, and most of its side effects are known to be mild and transient. Until now, coronary vasospasm has been rarely reported as a side effect of adenosine during or after adenosine stress test. This study reports a case of coronary vasospasm which was documented on stress myocardial perfusion CT imaging during adenosine stress test.
Adenosine is a direct coronary vasodilator that is widely used as a pharmacologic stress agent during myocardial perfusion imaging (1). Until now, a few cases of coronary vasospasm during or after completion of adenosine stress test have been reported (2, 3, 4, 5, 6, 7). To the best of our knowledge, there is no reported case in which coronary vasospasm was directly depicted on myocardial perfusion CT imaging during adenosine stress test. Herein, we report such a case. This study was approved by the Institutional Review Board.
A 73-year-old man visited the cardiovascular outpatient clinic for exertional substernal pain since 2 years, lasting three to four minutes. He had past medical history of diabetes mellitus and hypertension, and no history of coronary artery disease. His heart rate, blood pressure, and electrocardiography (ECG) were normal at rest. Initial serum creatine kinase-MB and Troponin-T concentrations were also within the normal range. Coronary CT angiography (CTA) and adenosine-stress myocardial perfusion CT were performed to identify coronary artery disease and presence of myocardial ischemia. The patient underwent cardiac CT using a 128-slice dual-source CT scanner (SOMATOM Definition Flash, Siemens Healthcare, Erlangen, Germany). The myocardial perfusion CT scan was performed using retrospective ECG-gated imaging with ECG-based tube current modulation and the MinDose technique (Siemens Healthcare). Maximum tube current was only applied during 30-80% of the R-R interval, and a tube current reduction of 4% was applied in the remaining heart phases. Scan parameters for stress myocardial perfusion CT were as follows: 2 × 128 × 0.6 mm detector collimation; 280 ms gantry rotation time; 100 kV tube voltage; and 280 mAs with the automatic tube current modulation technique.
The adenosine-stress myocardial perfusion CT scan was performed while adenosine (0.14 mg/kg/min) was being infused into the antecubital vein. Three minutes after the initiation of adenosine infusion, iodine-based contrast was also injected with 70 mL of contrast medium (iomeprol; Iomeron 400 mg I/mL; Bracco, Milan, Italy) at 4 mL/sec, followed by 40 mL of saline injection at the same rate. Immediately after stress myocardial perfusion CT, adenosine infusion was discontinued. After a rest period of 10 minutes and identification of the return of the patient's heart rate to baseline, a rest myocardial perfusion CT scan was obtained 1 minute after sublingual administration of 0.6 mg nitroglycerin. Scan parameters for rest myocardial perfusion CT were the same as those for stress myocardial perfusion CT.
In our patient, adenosine-stress myocardial perfusion CT showed mild to moderate decrease in perfusion of the subendocardial region in the mid to basal inferior and inferoseptal walls of the left ventricle (Fig. 1A). Coronary CTA revealed tight stenosis of the distal right coronary artery (RCA) (Fig. 1C) and no significant stenosis of the distal left circumflex artery (LCX) (Fig. 1D). There was no regional wall motion abnormality in the left ventricle. The extent of myocardial perfusion defect on the rest myocardial perfusion CT, when compared with that on the stress myocardial perfusion CT, was paradoxically increased in mid to basal inferior, inferoseptal, and inferolateral walls (Fig. 1B). Coronary CTA at rest showed increased extent of short segmental occlusion of the distal RCA (Fig. 1E), and newly developed segmental occlusion of the distal LCX (Fig. 1F). On the cine loop display, there was new development of mild hypokinesia in the RCA and LCX territories. The patient complained of chest discomfort during rest myocardial perfusion CT. However, his symptom disappeared quickly. Mean dose-length product for cardiac CT was 433 mGy cm and the effective dose was 6.0 mSv.
Conventional coronary angiography, which was performed on the following day, showed tight stenosis in the distal RCA, comparable to that on CTA performed during stress myocardial perfusion CT (Fig. 1G). Mild stenosis in the distal LCX was demonstrated (Fig. 1H). A coronary stent was implanted in the distal RCA.
Adenosine is a direct coronary vasodilator that is widely used as a pharmacologic stress agent during myocardial perfusion imaging. Its safety profile is well established and most of its side effects are known to be mild and transient (8, 9). However, coronary vasospasm, as a rare side effect of adenosine stress test, is an important cause of chest pain syndromes that can lead to myocardial infarction (10).
In the present case, the extent of myocardial perfusion defect on myocardial perfusion CT during the rest phase was paradoxically increased in the RCA territory, and CTA revealed segmental occlusion of the RCA. Moreover, there was new development of perfusion defect in the LCX territory on rest myocardial perfusion CT and segmental occlusion of the LCX on rest CTA, which were not depicted on stress myocardial perfusion CT. Subsequent conventional coronary angiography was negative for significant coronary artery disease in the LCX. Therefore, we think that coronary vasospasm, as a serious side effect of adenosine, could be the cause of this phenomenon.
The first choice of stress method during myocardial perfusion imaging is exercise, but in patients who cannot undergo the exercise stress, adenosine is a widely used alternative pharmaceutical stress method (11). The adenosine-stress myocardial perfusion CT has been recently introduced and has been combined with CTA, leading to improvement in the accuracy of cardiac CT for diagnosing coronary artery disease (12).
Coronary vasospasm has been reported rarely as a side effect of adenosine during or after adenosine stress perfusion test (2, 3, 4, 5, 6, 7). In the previous studies, patients developed chest pain with or without ECG abnormalities. To the best of our knowledge, this is the first report in which coronary vasospasm was directly depicted on coronary CTA and myocardial perfusion CT imaging during adenosine stress test. It is not well understood how adenosine causes coronary vasospasm. One possible mechanism may be activation of the adenosine A1 receptor that could cause contraction of the vascular smooth muscle, which has been confirmed in in vitro trials (10, 13). Another possible mechanism may be that discontinuation of a beta-blocker and calcium blocker may lead to elevated sympathetic tone (7). However, the exact mechanism of coronary vasospasm during or after adenosine stress test remains unclear. In our patient, coronary vasospasm could not be reversed immediately, regardless of administration of sublingual nitroglycerin. Coronary vasospasm is not always responsive to sublingual nitroglycerin, and drug-refractory coronary vasospasm is noted in approximately 20% of patients with coronary vasospasm (14).
In conclusion, although adenosine is a safe pharmacologic myocardial stress agent and adenosine-stress myocardial perfusion CT is helpful in the diagnosis of myocardial ischemia, the practitioner should be aware of this rare but possible complication, and hence close patient monitoring and careful assessment of all clinical symptoms and signs during and after the adenosine stress test are necessary.
Figures and Tables
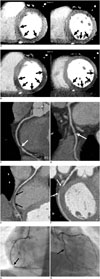 | Fig. 173-year-old man presenting with chest pain.Stress and rest myocardial perfusion CT images. A. Stress perfusion CT images in short-axis view showed mild to moderate subendocardial perfusion defect in mid to basal inferior and inferoseptal walls of left ventricle (arrows). B. Rest perfusion CT images in short-axis view showed increased extent of myocardial perfusion defect in mid to basal inferior, inferoseptal, and inferolateral walls of left ventricle (arrows). Stress CT angiography. Curved multiplanar reformatted images of RCA (C) and LCX (D) during stress showed tight stenosis of distal RCA (arrow in C) and no significant stenosis of distal LCX. Note stepping artifact in LCX (arrow in D). Rest CT angiography. Curved multiplanar reformatted images of RCA (E) and LCX (F) at rest demonstrated increased extent of segmental occlusion of distal RCA (arrows in E), and newly developed segmental occlusion of distal LCX (arrows in F). LCX = left circumflex artery, RCA = right coronary artery Conventional coronary angiography showed focal tight stenosis (arrow) in distal RCA (G) and mild stenosis in distal LCX (H). LCX = left circumflex artery, RCA = right coronary artery
|
References
1. Anagnostopoulos C, Harbinson M, Kelion A, Kundley K, Loong CY, Notghi A, et al. Procedure guidelines for radionuclide myocardial perfusion imaging. Heart. 2004; 90:Suppl 1. i1–i10.
2. Weissman G, Scandrett RM, Howes CJ, Russell RR 3rd. Coronary vasospasm during an adenosine stress test. J Nucl Cardiol. 2004; 11:747–750.
3. Faganello G, Belham M. Coronary vasospasm during an adenosine stress test. Int J Cardiol. 2006; 113:E84–E86.
4. Golzar J, Mustafa SJ, Movahed A. Chest pain and ST-segment elevation 3 minutes after completion of adenosine pharmacologic stress testing. J Nucl Cardiol. 2004; 11:744–746.
5. Rosenberg T, Perdrisot R. Coronary spasm after an adenosine stress test: an adverse effect of a vasodilator. Acta Cardiol. 2008; 63:401–404.
6. Nakayama M, Morishima T, Chikamori T, Aiga M, Takazawa K, Yamashina A. Coronary arterial spasm during adenosine myocardial perfusion imaging. J Cardiol. 2009; 53:288–292.
7. Han PP, Tian YQ, Wei HX, Wang Q, He ZX. Coronary spasm after completion of adenosine pharmacologic stress test. Ann Nucl Med. 2011; 25:580–585.
8. Cerqueira MD, Verani MS, Schwaiger M, Heo J, Iskandrian AS. Safety profile of adenosine stress perfusion imaging: results from the Adenoscan Multicenter Trial Registry. J Am Coll Cardiol. 1994; 23:384–389.
9. Baskot B, Rafajlovski S, Ristic´-Angelkov A, Obradovic´ S, Gligic´ B, Orozovic´ V, et al. [Study of efficacy and safety of pharmacological stress tests in nuclear cardiology]. Vojnosanit Pregl. 2009; 66:193–198.
10. Sato A, Terata K, Miura H, Toyama K, Loberiza FR Jr, Hatoum OA, et al. Mechanism of vasodilation to adenosine in coronary arterioles from patients with heart disease. Am J Physiol Heart Circ Physiol. 2005; 288:H1633–H1640.
11. Miyagawa M, Kumano S, Sekiya M, Watanabe K, Akutzu H, Imachi T, et al. Thallium-201 myocardial tomography with intravenous infusion of adenosine triphosphate in diagnosis of coronary artery disease. J Am Coll Cardiol. 1995; 26:1196–1201.
12. Feuchtner G, Goetti R, Plass A, Wieser M, Scheffel H, Wyss C, et al. Adenosine stress high-pitch 128-slice dual-source myocardial computed tomography perfusion for imaging of reversible myocardial ischemia: comparison with magnetic resonance imaging. Circ Cardiovasc Imaging. 2011; 4:540–549.
13. Ansari HR, Teng B, Nadeem A, Roush KP, Martin KH, Schnermann J, et al. A(1) adenosine receptor-mediated PKC and p42/p44 MAPK signaling in mouse coronary artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2009; 297:H1032–H1039.
14. Hung MJ, Hu P, Hung MY. Coronary artery spasm: review and update. Int J Med Sci. 2014; 11:1161–1171.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download