Abstract
A 53-year-old woman was referred for ventricular fibrillation with resuscitation. A CT-angiography showed signs of a right ventricular enlargement without obvious cause. A cardiac MRI demonstrated a dilated and hypokinetic right ventricle with extensive late gadolinium enhancement. Arrhythmogenic right ventricular dysplasia (ARVD) was suspected according to the "revised ARVD task force criteria". An endomyocardial biopsy was inconclusive. The patient developed purulent pericarditis after epicardial ablation therapy and died of toxic shock syndrome. The post-mortem pathologic examination demonstrated sarcoidosis involving the heart, lungs, and thyroid gland.
Sarcoidosis is an idiopathic granulomatous disease characterized by the presence of non-caseating granulomas in affected tissues. The lungs are most often involved, but it may affect any organ, particularly the heart, skin, eyes, reticuloendothelial system, kidneys, and central venous system (1, 2). Postmortem studies indicate that cardiac involvement ranges from 20% to 25%, with higher prevalence in Japan and Scandinavia (2, 3). Clinically, the cardiac involvement is most often silent and becomes apparent in only about 5% of patients. Symptoms of cardiac sarcoidosis are nonspecific and depend on the location and extent of the granulomatous inflammation. This may include conduction abnormalities with bundle branch block or atrioventricular block (25-30%), ventricular arrhythmias (23%, ranging from isolated premature ventricular beats to sustained or non-sustained ventricular tachycardia [VT]), and even ventricular fibrillation that leads to sudden death (35%) (3). Cardiac sarcoidosis may also cause systolic or diastolic heart failure with poor prognosis (1). Non-caseating granulomatous infiltration and fibrosis are likely to be located in the left ventricle (LV), but right ventricle (RV) free wall involvement may also be present in up to 40% of the cases. RV involvement may mimic arrhythmogenic right ventricular dysplasia (ARVD) on an electrocardiographic (ECG) and image findings. ARVD is a rare genetic condition characterized by fibro-fatty tissue infiltration, ventricular dysfunction, and arrhythmias, typically of the RV. Rarely is the LV involved (4). Differentiation between sarcoidosis and ARVD can be especially challenging with biventricular involvement while differentiation is important with regard to therapeutic options and prognosis. In this paper, we presented a patient who was diagnosed as having ARVD on the basis of clinical symptoms and ECG and image findings while postmortem examination showed cardiac sarcoidosis.
Our Institutional Review Board declared that formal ethical approval for a retrospective analysis of clinically obtained data in this report was not needed. Patient-informed consent was obtained from the relatives.
A 53-year-old woman was referred to our institution after a ventricular fibrillation and a successful resuscitation. Thoracic CT, performed to rule out pulmonary embolism, showed a RV enlargement without obvious cause (Fig. 1A). Pulmonary pathology was not present except for bilateral dependent atelectasis. A few slightly enlarged mediastinal lymph nodes were observed but were not suspected (Fig. 1A). A cardiac MRI showed a dilated RV with global hypokinesia and an end-diastolic volume of 130 mL/m2 (normal value: 67 ± 10 mL/m2; 95% confidence interval [CI], 48-87 mL/m2) (5), with an ejection fraction of 24.5% (normal value: 63 ± 8%; 95% CI, 47-80%) (5). Dyskinesia of the interventricular septum was also noted. The LV morphology and function were normal (ejection fraction, 63%), and pericardial effusion was not observed. Extended late gadolinium enhancement (LGE) was present in large parts of the RV free wall, RV outflow tract, and moderator band, while limited patchy LGE was present in the apical interventricular septum and basal inferolateral LV wall (Fig. 1B).
Both mediastinum and lungs were unremarkable except for a minimal right-sided pleural effusion. ARVD was suspected according to the MRI findings and the revised ARVD task force criteria (6). Five months later, the patient was readmitted with an electrical storm unresponsive to drug therapy. An endocardial catheter ablation was not successful, most likely due to a subepicardial substrate for induced VT. The patient was scheduled for an epicardial ablation. A cardiac CT, performed prior to epicardial mapping, showed normal coronary arteries and no pulmonary or mediastinal abnormalities. A clinical genetic analysis did not show the typical abnormalities associated with ARVD. An endomyocardial biopsy of the RV was performed, but the histologic findings were inconclusive. Lysozyme levels increased and clinically suggested sarcoidosis (1042 µg/L; reference value, 240-560 µg/L). However, imaging features met the criteria for ARVD (6). An epicardial mapping confirmed a subepicardial substrate, which was targeted by a catheter ablation. The patient developed purulent pericarditis caused by an enterotoxic S. aureus two days after the epicardial ablation therapy and died of toxic shock syndrome within 24 hours. The post-mortem examination demonstrated massive sarcoidosis involving the heart, both lungs, and the thyroid gland. Enlarged lymph nodes were not found. Macroscopically, extended areas of discoloration were observed in the myocardium. These areas included the RV, extensively, and focal areas in the LV and the interventricular septum, with the same distribution of LGE on the MRI (Fig. 1C). Microscopic examination revealed a non-caseating granulomatous inflammation, containing epithelioid histiocytes, multinucleated giant cells, and fibrosis. There was no myocyte necrosis and there were occasional eosinophils. Close to the granulomas, a sparse lymphocytic infiltration was present (Fig. 1D). These characteristics were diagnostic for cardiac sarcoidosis (7).
In the literature, there is an increasing number of case reports and series reporting sarcoidosis with predominant RV involvement. This usually presents with arrhythmia and morphological or functional RV abnormalities, generally in association with other clinical or radiological findings that pointed to a sarcoidosis diagnosis, such as enlarged mediastinal lymph nodes or pulmonary involvement (8, 9, 10). Vasaiwala et al. (9) reported on 20 consecutive patients with suspected ARVD. Three of the 20 patients (15%) were found to have non-caseating granulomas on biopsy, consistent with cardiac sarcoidosis. It is noteworthy that patients with histopathologic diagnosis of ARVD were no different from patients with sarcoidosis granulomas, regarding most clinical variables, such as age, symptoms, ECG abnormalities, late potentials, arrhythmias, or findings on a chest radiography or MRI. The study demonstrated that cardiac sarcoidosis may account for an ARVD phenotype in an unexpectedly high proportion of cases. The only variable that was found significantly associated with cardiac sarcoidosis was LV dysfunction with an ejection fraction below 50% (3 of 3 sarcoidosis patients versus 2 of 17 ARVD patients). The authors suggested that LV function could be useful in the differential diagnosis, as LV dysfunction is more likely present in cardiac sarcoidosis than in ARVD (9). In our patient, normal LV function and, especially, the absence of extracardiac manifestations of sarcoidosis, both clinically and radiologically, suggested the diagnosis of ARVD with biventricular involvement. ARVD and sarcoidosis with biventricular involvement may have similar LGE patterns. In both cases, patchy enhancement is usually transmural in the RV and subepicardial and/or midwall in the LV. In ARVD, LGE can be present in up to a third of patients (11, 12) and is usually located in the RV free wall, in the outflow tract, and in the posterior/inferior wall. Enhancement is associated with scar tissue (12). In sarcoidosis, cardiac LGE is present in up to a fourth of patients (13). LGE distribution is usually subepicardial and/or midwall, and commonly involved locations are the RV side of the interventricular septum and the LV basal anterolateral and anteroseptal regions (14). Transmural lesions are associated with poor LV ejection fraction (15). In the literature, we found only one case of clinically isolated cardiac sarcoidosis similar to our patient (10). To the best of our knowledge, there are no other cases that demonstrate the exact correlation between MRI and pathology examinations of cardiac sarcoidosis. It is important to be aware that sarcoidosis can mimic ARVD and to take into consideration the differential diagnosis cardiac sarcoidosis, even when the revised ARVD task force criteria are fulfilled.
Figures and Tables
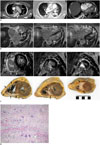
Fig. 1
Cardiac sarcoidosis mimicking arrhythmogenic right ventricular dysplasia in 53-year-old woman.
A. Pulmonary CT angiography on admission. Slightly enlarged mediastinal lymph nodes, aspecific finding (arrows, left panel). Bilateral dependent atelectasis but no other pulmonary abnormalities and no pulmonary embolism (left and middle panel). Enlarged right heart, with bowing of interventricular septum towards LV (right panel). B. MRI in axial orientation. Upper level (left panel), middle level (middle panel), and lower level (right panel) MRI showing bright LGE (arrows) in large parts of RV and patchy subepicardial LGE in interventricular septum and LV. LGE = late gadolinium enhancement, LV = left ventricle, RV = right ventricle C. Short-axis MRI images (upper row) compared with gross anatomic sections (lower row). Note perfect correlation between high signal LGE spots (arrows) on MRI at RV wall and apical septum and sarcoidosis granuloma (arrows) on pathology. Base (left panels), mid-ventricular (middle panels), and apex (right panels). D. Histology showing infiltration of histiocytes between myocardial fibers with formation of confluent granulomas and multinucleated giant cells, diagnostic for sarcoidosis (hematoxylin and eosin stain, × 100). Fibrosis and sparse lymphocytic infiltration are present. LGE = late gadolinium enhancement, LV = left ventricle, RV = right ventricle
References
1. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007; 357:2153–2165.
2. Mantini N, Williams B Jr, Stewart J, Rubinsztain L, Kacharava A. Cardiac sarcoid: a clinician's review on how to approach the patient with cardiac sarcoid. Clin Cardiol. 2012; 35:410–415.
3. Roberts WC, McAllister HA Jr, Ferrans VJ. Sarcoidosis of the heart. A clinicopathologic study of 35 necropsy patients (group 1) and review of 78 previously described necropsy patients (group 11). Am J Med. 1977; 63:86–108.
4. Corrado D, Basso C, Thiene G, McKenna WJ, Davies MJ, Fontaliran F, et al. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. 1997; 30:1512–1520.
5. Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP Jr. Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson. 1999; 1:7–21.
6. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010; 121:1533–1541.
7. Lagana SM, Parwani AV, Nichols LC. Cardiac sarcoidosis: a pathology-focused review. Arch Pathol Lab Med. 2010; 134:1039–1046.
8. Yared K, Johri AM, Soni AV, Johnson M, Alkasab T, Cury RC, et al. Cardiac sarcoidosis imitating arrhythmogenic right ventricular dysplasia. Circulation. 2008; 118:e113–e115.
9. Vasaiwala SC, Finn C, Delpriore J, Leya F, Gagermeier J, Akar JG, et al. Prospective study of cardiac sarcoid mimicking arrhythmogenic right ventricular dysplasia. J Cardiovasc Electrophysiol. 2009; 20:473–476.
10. Ladyjanskaia GA, Basso C, Hobbelink MG, Kirkels JH, Lahpor JR, Cramer MJ, et al. Sarcoid myocarditis with ventricular tachycardia mimicking ARVD/C. J Cardiovasc Electrophysiol. 2010; 21:94–98.
11. Hunold P, Wieneke H, Bruder O, Krueger U, Schlosser T, Erbel R, et al. Late enhancement: a new feature in MRI of arrhythmogenic right ventricular cardiomyopathy? J Cardiovasc Magn Reson. 2005; 7:649–655.
12. Santangeli P, Hamilton-Craig C, Dello Russo A, Pieroni M, Casella M, Pelargonio G, et al. Imaging of scar in patients with ventricular arrhythmias of right ventricular origin: cardiac magnetic resonance versus electroanatomic mapping. J Cardiovasc Electrophysiol. 2011; 22:1359–1366.
13. Cain MA, Metzl MD, Patel AR, Addetia K, Spencer KT, Sweiss NJ, et al. Cardiac sarcoidosis detected by late gadolinium enhancement and prevalence of atrial arrhythmias. Am J Cardiol. 2014; 113:1556–1560.
14. Ordovas KG, Higgins CB. Delayed contrast enhancement on MR images of myocardium: past, present, future. Radiology. 2011; 261:358–374.
15. Watanabe E, Kimura F, Nakajima T, Hiroe M, Kasai Y, Nagata M, et al. Late gadolinium enhancement in cardiac sarcoidosis: characteristic magnetic resonance findings and relationship with left ventricular function. J Thorac Imaging. 2013; 28:60–66.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download