Abstract
We describe a rare case of extralobar pulmonary sequestration with hemorrhagic infarction in a 10-year-old boy who presented with acute abdominal pain and fever. In our case, internal branching linear architecture, lack of enhancement in the peripheral portion of the lesion with internal hemorrhage, and vascular pedicle were well visualized on preoperative magnetic resonance imaging that led to successful preoperative diagnosis of extralobar pulmonary sequestration with hemorrhagic infarction probably due to torsion.
Pulmonary sequestration is a congenital pulmonary malformation in which non-functioning lung tissue receives systemic arterial blood supply and does not communicate with the adjacent tracheobronchial tree. To establish the diagnosis of the anomaly and its type, the feeding artery, draining vein, and pleural lining should be evaluated with ultrasonography (US), CT, or MRI. In contrast to the more common intralobar type which shares its visceral pleura with adjacent lung tissue and has pulmonary venous drainage, the less common extralobar type has its own visceral pleura and systemic venous drainage. In more than half of the cases, the extralobar type is often associated with other congenital anomalies, such as bronchopulmonary foregut malformation and congenital diaphragmatic hernia. The intralobar type may be complicated with infection, while the extralobar type is usually asymptomatic, and thus almost all cases are discovered on antenatal ultrasonographic screening or incidentally. However, an extralobar pulmonary sequestration may rarely undergo torsion of its vascular pedicles, resulting in infarction. To the best of our knowledge, only nine pediatric cases of extralobar pulmonary sequestration with hemorrhagic infarction have been reported (1, 2, 3, 4, 5, 6, 7, 8, 9). Preoperative imaging diagnosis of pulmonary malformation was made in one of these cases (5), and preoperative diagnosis of complicated infarction could not be made in all of the cases. Therefore, we report the preoperative imaging findings of extralobar pulmonary sequestration with hemorrhagic infarction in a child with pathological correlation. The case report was approved by our Institutional Review Board.
A previously healthy 10-year-old boy presented with left upper quadrant abdominal pain that aggravated by deep breathing or lying down and fever. He also complained of mild cough and whitish sputum. On physical examination, the child was irritable; but there were no other remarkable findings. Laboratory data showed elevated C-reactive protein (8.65 mg/dL) without leukocytosis and other findings were unremarkable.
A chest radiograph showed a left lower lobe retrocardiac opacity with left pleural effusion. Chest and abdominal CT performed elsewhere with 4 mm-thick axial slices and 3 mm-thick coronal and sagittal reformatted slices revealed an approximately 3 × 3 × 4 cm-sized, well-defined heterogeneously hypodense soft tissue lesion in the left pleural space with pleural effusion and atelectasis of the adjacent lung and no other remarkable abdominal findings. No feeding artery or draining vein was identified on CT.
Chest US using a convex, low-frequency (3.5 MHz) transducer showed a lentiform, heterogeneously echogenic solid mass with hypoechoic linear branching structures in the left lower hemithorax, sharply demarcated from the adjacent atelectatic lung and partly abutting the descending aorta (Fig. 1A). The mass showed minimal vascularity on color Doppler US (Fig. 1B). A large amount of left pleural effusion containing low-level echoes was also identified. Based on the sonographic findings, pulmonary malformation was suspected. US-guided thoracentesis was subsequently performed with cytologic evaluation of the aspirated pleural fluid. The aspirated fluid was a bloody exudate (red blood cell 940000/mm3, white blood cell 15790/mm3, neutrophils 39%, lymphocytes 15%, histiocytes 38%, mesothelial cells 6%, atypical cells 2%, protein 4.6 g/dL, glucose 109 mg/dL, lactate dehydrogenase 504 U/L, amylase 31 U/L, lipase 15 U/L, adenosine deaminase 23.4 U/L). The pleural fluid culture showed no growth of infectious organisms.
The same mass lesion was seen on chest MRI using a 1.5-T scanner. The mass showed heterogeneous hyperintensity suggesting a hemorrhagic component on T1-weighted imaging (6-mm slice thickness, 25-cm field of view, and imaging matrix of 512 × 512), and round, linear, and branching hyperintense foci on T2-weighted imaging (6-mm slice thickness, 27-cm field of view, and imaging matrix of 512 × 512) (Fig. 1C). Of interest, a small tubular signal void arising from the descending aorta and heading toward the lesion suggestive of a feeding artery was seen and the hemiazygos vein that deemed to be a draining vein was found to be mildly dilated (Fig. 1C, D). MR angiography was not performed because a feeding artery was not delineated with contrast-enhanced CT using higher spatial resolution. To further characterize the lesion perfusion, dynamic contrast-enhanced T1-weighted imaging (5-mm slice thickness, 23-cm field of view, and imaging matrix of 224 × 224) was performed and the lesion showed irregular, heterogeneous, delayed enhancement in the center and decreased or lack of contrast enhancement in the periphery (Fig. 1E). The probable diagnosis based on the imaging findings, particularly the MRI findings, was extralobar pulmonary sequestration complicated with hemorrhagic infarction.
The patient subsequently underwent a planned operation for complete excision of the mass. Extralobar pulmonary sequestration was also suspected at surgery and a small feeding vessel arising from the thoracic aorta was identified and clipped. On gross pathological examination, the mass was dark brown, lobulated, and myxoid with a large tubular structure in the center (Fig. 1F). The histopathologic examination confirmed the diagnosis of extralobar pulmonary sequestration with hemorrhagic infarction and anomalous lung tissue with the cartilages (Fig. 1G, H). Hemorrhagic infarction of the lesion was presumed to be caused by torsion based on the acute clinical course, although torsion of a vascular pedicle was not definitely identified at surgery. The patient recovered well postoperatively without complications.
Extralobar pulmonary sequestration is mostly detected in childhood and rarely detected in adulthood. Almost all cases of extralobar pulmonary sequestration are asymptomatic and a complication such as an infarction has been reported very rarely. Besides two adult patients, nine pediatric cases of extralobar pulmonary sequestration with torsion have been reported in the literature (Table 1) (1, 2, 3, 4, 5, 6, 7, 8, 9). These cases included five boys and four girls with an age range of 4-13 years (median age, 10 years). Abdominal pain was present in all of the patients, and chest pain, dyspnea, or flank tenderness was present in some of the patients. Elevated inflammatory blood markers, such as leukocytosis, C-reactive protein, and erythrocyte sedimentation rate, were present in two patients. The age of our patient was equal to the median age in the reported cases, and our patient also presented with abdominal pain and elevated C-reactive protein.
Radiologically, extralobar pulmonary sequestration without infarction manifests as a single, sharply marginated, homogeneous soft tissue mass usually located in the lower hemithorax in close association with the posterior medial hemidiaphragm. Initially, a systemic feeding artery and a systemic draining vein (less frequently) are often detected on color Doppler US, contrast-enhanced CT, or MRI.
In cases complicated with hemorrhagic infarction, chest radiography was nonspecific, demonstrating lower lobe opacity and/or pleural effusion. Cross-sectional imaging features (US, CT, MRI) of extralobar pulmonary sequestration are summarized in Table 1. All lesions were located in the medial basal segment (lower paraspinal region), and the majority of lesions were located in the left side (88.9%, 8/9). The lesion in our case was also located in the left medial basal segment. In all ten cases including our case, imaging evaluation showed a polygonal or well-circumscribed mass with pleural effusion. Pleural effusion was often hemorrhagic and complicated by torsion and infarction; thus internal echoes were seen in pleural effusion on US and pleural enhancement was seen on contrast-enhanced CT or MRI. In our case, the internal architecture of the lesions that were proven to be malformed bronchioles was hardly seen on CT but it was more easily detected on US or MRI. The identification of the internal architecture on cross-sectional imaging appears to be quite helpful in suggesting the diagnosis of pulmonary sequestration. In fact, this feature was not described in one case even though it was clearly seen on MRI (2). In addition, the dilated lymphatics may be seen as cystic structures in the lesion probably as a result of obstructed venous and efferent lymphatic drainage caused by torsion, which may explain the development of pleural effusion and tension hydrothorax in these patients. Therefore, it was suggested as an indirect helpful sign for diagnosing torsion of the extralobar pulmonary sequestration (10). However, dilated lymphatics were not clearly seen in our case on histologic examination.
In our case, the lesion showed heterogeneous central enhancement with decreased or lack of enhancement in the peripheral portion on dynamic contrast-enhanced MRI. The decreased or lack of enhancement in the peripheral portion can be the direct sign of infarction in our case. On the other hand, heterogeneous central enhancement on dynamic contrast-enhanced MRI in conjunction with minimally increased vascularity on color Doppler US is unusual for infarction and has not been reported in this rare condition. We postulate that this unusual finding in our case is due to repeated, intermittent events of partial occlusion and subsequent recanalization. Gawlitza et al. (2) also suggested that the lack of contrast enhancement in the lesion is an imaging sign of torsion of pulmonary sequestration, together with no visible feeding vascular pedicle. Non-identifiable feeding vessel probably due to thrombosis (2, 10) was actually described as one of the imaging findings suggestive of infarcted extralobar pulmonary sequestration. In our case, a feeding artery and a draining vein were identified as signal-void vascular structures on MRI but not on contrast-enhanced CT. This false-negative CT finding may be explained by the fact that the acquisition time of contrast-enhanced CT seems to be too early to visualize the already compromised small vessels, and it strongly indicates that a delayed phase is necessary to detect the vascular pedicle. The presence of the vascular pedicle was confirmed at surgery in our case. As in uncomplicated pulmonary sequestration, presence of the vascular pedicle in a preoperative imaging study would be a critical hint to make the diagnosis of complicated pulmonary sequestration as in the present case. In the previously reported nine cases, a feeding artery or a draining vein was not identified on imaging studies but a feeding artery was usually (88.9%, 8/9) detected at surgery (Table 1).
In summary, we can suggest extralobar pulmonary sequestration complicated with hemorrhagic infarction as the most probable diagnosis, based on the following imaging findings: 1) typical location of pulmonary sequestration, 2) visceral pleural lining showing a polygonal and well-defined mass, 3) linear or branching internal architecture, 4) feeding artery and draining vein, 5) lack of enhancement in the periphery, and 6) internal hemorrhage. It should be noted that the failure to identify the above mentioned imaging findings might delay the correct diagnosis, and it frequently led to misdiagnosis in the previously reported cases (1, 2, 3, 4, 5, 6, 7, 8, 9).
We have described rare but characteristic US, CT, and MRI findings in a child with torsion of the extralobar pulmonary sequestration and hemorrhagic infarction. In our case, internal architecture demonstrated on US and MRI favors the diagnosis of malformed tissue rather than neoplasm. The vascular pedicle exclusively seen on MRI obviously increased our diagnostic confidence in pulmonary sequestration. The lack of enhancement in the peripheral portion of the lesion and internal hemorrhage were the important clues that suggest hemorrhagic infarction proven by histopathology.
Figures and Tables
Fig. 1
10-year-old boy with extralobar pulmonary sequestration with hemorrhagic infarction.
A. Left posterior sagittal chest ultrasonography using convex, low-frequency (3.5 MHz) transducer shows lentiform heterogeneous solid mass (arrowheads) with linear structures (arrow) in left lower hemithorax. Adjacent consolidated lung and large amount of left pleural effusion containing low-level echoes were also identified. B. Color Doppler ultrasonography demonstrates minimal vascularity in lesion. C. Axial T2-weighted image shows well-defined triangular solid mass lesion with left pleural effusion. Central branching cystic structure (arrow) within mass corresponded to dilated bronchiole on histopathologic examination. Hemiazygos vein (arrowhead) appears to be mildly dilated. D. Axial T1-weighted image reveals small artery arising from descending thoracic aorta (arrows) and connecting to lesion. E. Dynamic contrast-enhanced image demonstrates irregular central enhancement with several foci of poor enhancement (arrows) in periphery of lesion. Pleural enhancement is also noted. F. Cut section of excised lesion shows dark brown triangular mass with 0.5 cm-sized central tubular structure (arrow). Photomicrographs (G, H) show hemorrhagic infarction with ample red blood cells (G) and anomalous lung tissue with cartilages (asterisks in H) (hematoxylin and eosin stain, × 50).
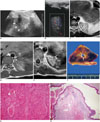
Table 1
Summary of Imaging and Operative Findings of 10 Cases

| No. | Authors |
Age (Years) |
Sex | Location | Pleural Effusion | US/Color Doppler Findings | CT Findings | MRI Findings | Preoperative Diagnosis | Feeding Vessel on Imaging | Feeding Vessel at Surgery |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chen et al. (1) | 13 | M | Left lower medial basal | + | Mass/NA | Well-defined non-calcified mass | NA | Neurogenic tumor | - | + |
| 2 | Huang et al. (3) | 13 | F | Left lower medial basal | + | NA/NA | Solid mass | Hypointense mass | NA | - | - |
| 3 | Shah et al. (7) | 11 | F | Left lower medial basal | + | NA/NA | Ovoid mildly enhancing heterogeneous soft-tissue mass; pleural enhancement | NA | NA | - | + |
| 4 | Zucker et al. (9) | 6 | M | Left lower medial basal | + | Homogeneously echogenic solid mass/no vascularity | Oval-shaped soft tissue mass with two punctate calcifications | NA | NA | - | + |
| 5 | Uchida et al. (8) | 4 | M | Left lower medial basal | + | Echogenic, well-circumscribed mass/no vascularity | Circumscribed, ovoid mass | NA | NA | - | + |
| 6 | Mammen et al. (6) | 8 | F | Right lower medial basal | + | Complex-echo lesion/NA | NA | NA | Pneumonia with parapneumonic pleural effusion | - | + |
| 7 | Lima et al. (5) | 11 | F | Left lower medial basal | + | NA/NA | Mass | Mass | Pulmonary malformation | - | + |
| 8 | Gawlitza et al. (2) | 11 | M | Left lower medial basal | + | Sharply demarcated mass/NA | Non-calcified soft tissue mass | Polygonal non-enhancing hypointense mass | Extralobar pulmonary sequestration | - | + |
| 9 | Kirkendall and Guiot (4) | 13 | M | Left lower medial basal | + | NA/NA | Enlarged non-calcified soft tissue mass | NA | NA | - | + |
| 10 | Choe and Goo (our case) | 10 | M | Left lower medial basal | + | Sharply demarcated echogenic solid mass with hypoechoic linear branching structures/minimal vascularity | Well-defined heterogeneously hypodense soft tissue lesion | Hemorrhagic mass with round, linear, and branching foci; mildly dilated hemiazygos vein; delayed enhancement in the center and decreased or lack of contrast enhancement in the periphery; pleural enhancement | Torsed extralobar pulmonary sequestration with hemorrhagic infarction | + (MRI) | + |
References
1. Chen W, Wagner L, Boyd T, Nagarajan R, Dasgupta R. Extralobar pulmonary sequestration presenting with torsion: a case report and review of literature. J Pediatr Surg. 2011; 46:2025–2028.
2. Gawlitza M, Hirsch W, Weißer M, Ritter L, Metzger RP. Torsion of extralobar lung sequestration - lack of contrast medium enhancement could facilitate MRI-based diagnosis. Klin Padiatr. 2014; 226:38–39.
3. Huang EY, Monforte HL, Shaul DB. Extralobar pulmonary sequestration presenting with torsion. Pediatr Surg Int. 2004; 20:218–220.
4. Kirkendall ES, Guiot AB. Torsed pulmonary sequestration presenting with gastrointestinal manifestations. Clin Pediatr (Phila). 2013; 52:981–984.
5. Lima M, Randi B, Gargano T, Tani G, Pession A, Gregori G. Extralobar pulmonary sequestration presenting with torsion and associated hydrothorax. Eur J Pediatr Surg. 2010; 20:208–210.
6. Mammen A, Myers NA, Beasley SW. Torsion and infarction of an extralobar pulmonary sequestration. Pediatr Surg Int. 1994; 9:399–400.
7. Shah R, Carver TW, Rivard DC. Torsed pulmonary sequestration presenting as a painful chest mass. Pediatr Radiol. 2010; 40:1434–1435.
8. Uchida DA, Moore KR, Wood KE, Pysher TJ, Downey EC. Infarction of an extralobar pulmonary sequestration in a young child: diagnosis and excision by video-assisted thoracoscopy. J Laparoendosc Adv Surg Tech A. 2010; 20:399–401.
9. Zucker EJ, Tracy DA, Chwals WJ, Solky AC, Lee EY. Paediatric torsed extralobar sequestration containing calcification: Imaging findings with pathological correlation. Clin Radiol. 2013; 68:94–97.
10. Takeuchi K, Ono A, Yamada A, Toyooka M, Takahashi T, Shigematsu Y, et al. Two adult cases of extralobar pulmonary sequestration: a non-complicated case and a necrotic case with torsion. Pol J Radiol. 2014; 79:145–149.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download